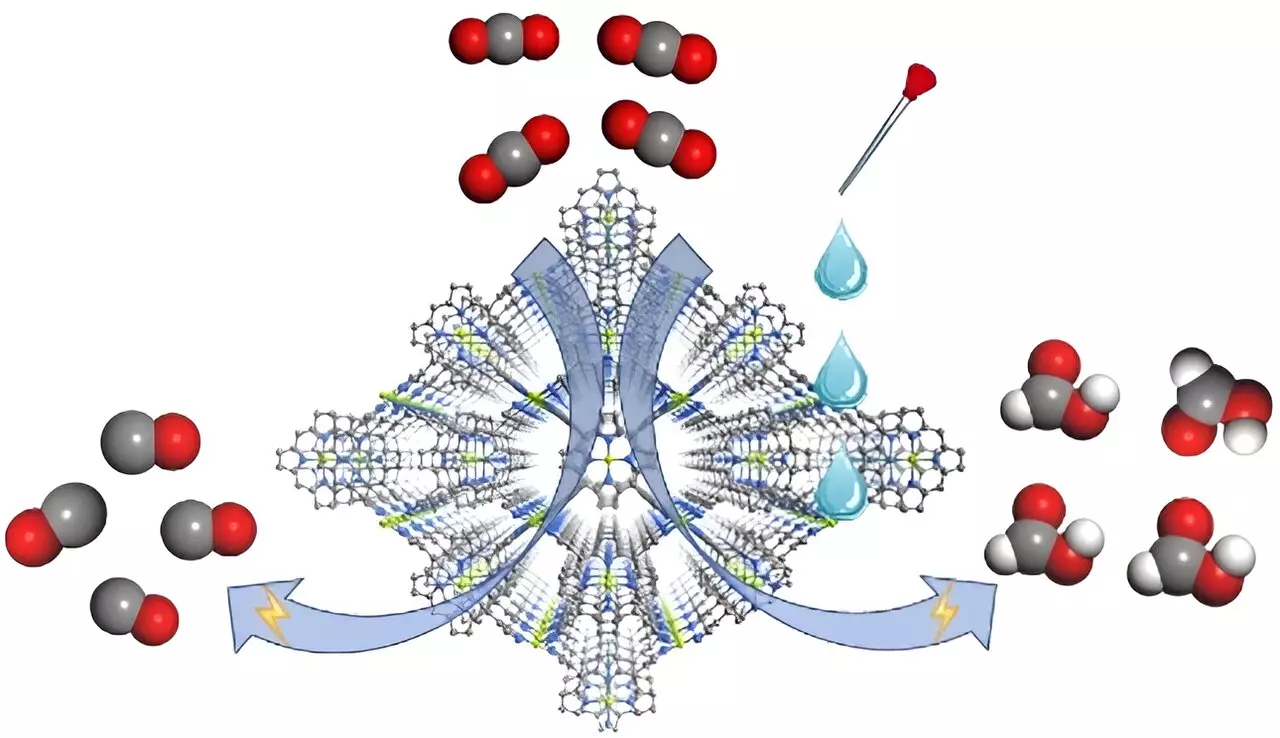
The electrochemical reduction of carbon dioxide (CO2) represents a frontier in sustainable energy research, as scientists seek viable methods to recycle this greenhouse gas into useful chemicals. While significant progress has been made in understanding catalyst design and optimization, less attention has been given to the electrolyte’s composition – an oversight that impacts product selectivity substantially. New findings have emerged from a team of researchers led by Prof. Cao Rong and Prof. Zhang Teng, who delve into the nuanced interplay between electrolyte composition and catalyst functionality, bringing forward a metal-organic framework (MOF) catalyst named FICN-8.
FICN-8 distinguishes itself through its sophisticated architecture composed of Cu(porphyrin) and Cu(pyrazolate) units, fostering a three-dimensional porous structure. This particular design permits maximal accessibility of the catalytic sites within the metalloporphyrin framework. Innovatively, this catalyst can be adjusted through a variety of solvent or electrolyte compositions, which is pivotal for enhancing the efficiency of electrochemical reactions. During experimentation, FICN-8 demonstrated remarkable effectiveness in facilitating the reduction of CO2, showcasing an impressive 95% selectivity for CO when paired with a TBAPF6/acetonitrile electrolyte.
One of the significant revelations of this research revolves around the influence of the electrolyte composition on product selectivity. When additional water or trifluoroethanol (TFE)—acting as proton donors—are introduced into the system, the primary product shifts from CO to formic acid. Specifically, the research reports a notable increase in Faradaic efficiency for formic acid, peaking at 48% with precise concentrations of water or TFE. This finding not only underscores the electrolyte’s paramount role in steering the reaction pathway but illustrates the potential for designing adaptive systems aimed at producing high-value chemicals from CO2.
To bolster their findings, the researchers undertook a comprehensive approach to elucidate the underlying mechanisms that govern this selectivity. Through kinetic isotope effect (KIE) studies, they noted that the formation of formic acid involves direct proton participation, evidenced by a high KIE value (3.7±0.7). In contrast, CO formation seemingly occurs via a distinct pathway that diminishes the influence of proton concentration. Theoretical modeling further corroborates these observations, identifying a critical step involving the reductive adsorption of hydride at the porphyrin’s nitrogen site, essential for formic acid production.
This research unveils a pivotal understanding of how electrolyte compositions can significantly modulate product outcomes in electrochemical CO2 reduction processes. By employing FICN-8, the study proposes a pathway toward the integration of novel catalyst-electrolyte systems that could accelerate the transformation of CO2 into economically advantageous compounds. In light of the urgency surrounding climate change, continued exploration of these tactics will be essential in developing efficient strategies that capitalize on CO2, transforming a liability into a resource. Advancements in this field may not only facilitate reductions in atmospheric CO2 but potentially pioneer new industries based on carbon recycling technologies.

Leave a Reply