Hydrogen (H2) has long been recognized as a promising fuel for reducing greenhouse gases, particularly when produced using renewable energy sources to split water molecules (H2O). However, the process of breaking water into hydrogen and oxygen is more complex than it may initially appear. This complexity stems from the need for catalysts to facilitate two simultaneous electrochemical reactions crucial for this transformation. Recently, scientists at the U.S. Department of Energy’s Brookhaven National Laboratory and Columbia University have made significant strides in developing a more efficient catalyst for the challenging oxygen evolution reaction, a critical step in the production of hydrogen through water electrolysis.
Challenges in Catalyst Development
One of the primary obstacles to widespread adoption of hydrogen as a clean fuel is the reliance on expensive and rare metals, such as iridium, for catalytic purposes. Iridium, while effective, is costly and scarce, posing a barrier to large-scale production. The goal of the research conducted by the team was to reduce the amount of iridium required for efficient catalysis without compromising performance. By leveraging theoretical calculations to guide their approach, the scientists sought to design a catalyst that maximized efficiency and stability in acidic conditions, where the oxygen evolution reaction takes place.
Designing the New Catalyst
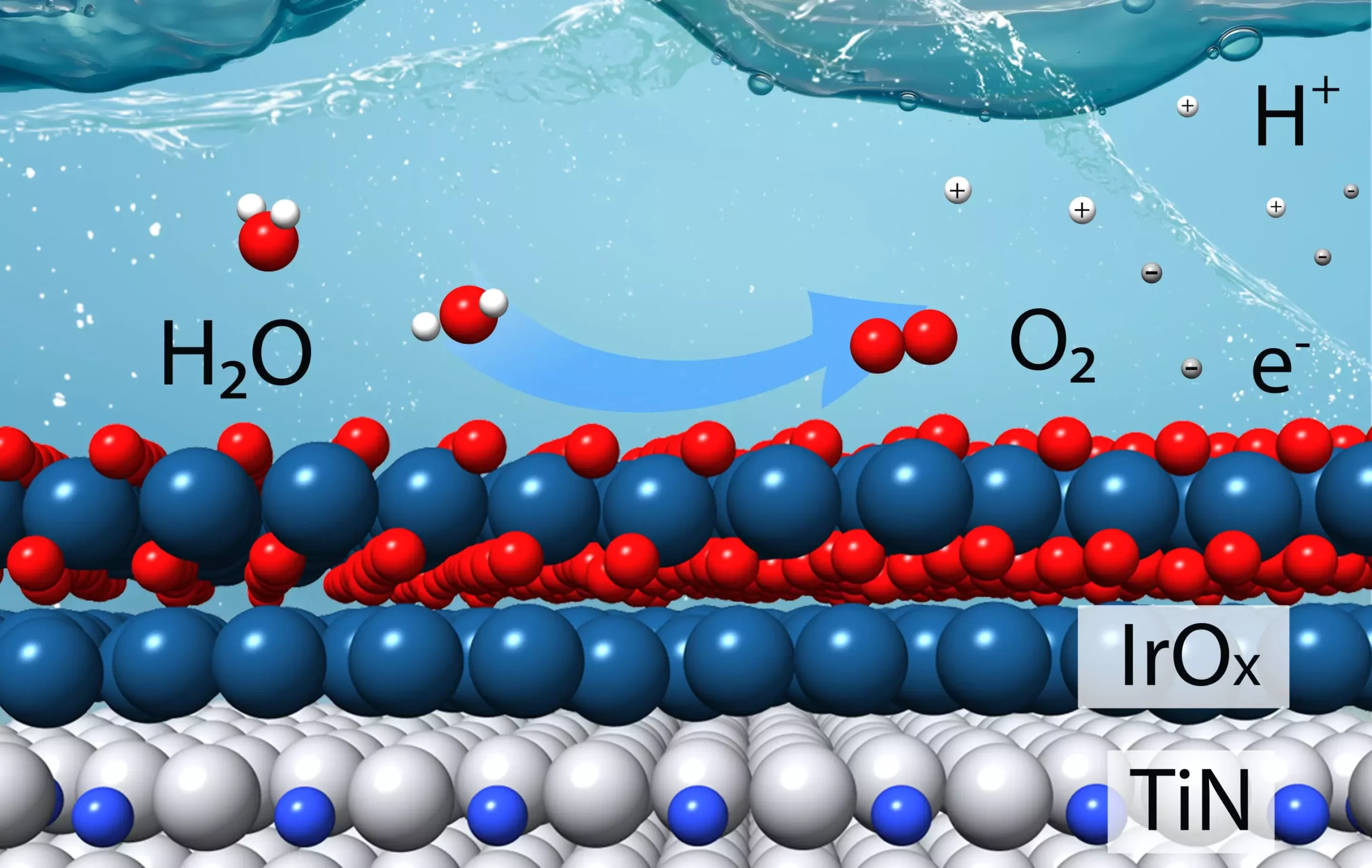
The catalyst developed by the research team was meticulously crafted based on a bottom-up approach inspired by theoretical modeling. By combining titanium with nitrogen to form stable titanium nitrides, the researchers aimed to create a core material that could be coated with a thin layer of iridium. Through density functional theory calculations, the team determined that multiple layers of iridium would enhance the catalyst’s performance and stability. Experimental validation of the theoretical predictions confirmed that the new catalyst, composed of a titanium nitride core with two to three layers of iridium, exhibited superior efficiency compared to commercially available iridium catalysts.
To evaluate the effectiveness of the catalyst, the team conducted a series of experiments using thin films and powdered samples resembling industrial applications. Advanced techniques such as transmission electron microscopy and X-ray spectroscopy were employed to analyze the catalyst’s structure and interactions at the atomic level. The studies revealed that the iridium-titanium bonding promoted stability and optimized catalytic activity by enhancing the binding of reaction intermediates. The charge transfer between the titanium nitride core and the iridium surface played a crucial role in fine-tuning the catalyst’s performance, highlighting the importance of material interactions at the nanoscale.
While the new catalyst shows great promise in laboratory settings, the challenge lies in transitioning towards large-scale production to enable the widespread adoption of green hydrogen technology. Current efforts are focused on addressing the scalability of catalyst manufacturing and optimizing the consistency of the catalytic powders. By refining production techniques to achieve uniform core-shell structures with a thin and continuous iridium layer, researchers aim to enhance the cost-effectiveness of water electrolysis and pave the way for the commercial production of green hydrogen on a significant scale.
The development of a more efficient catalyst for the oxygen evolution reaction represents a significant advancement in the quest for sustainable hydrogen production. Through a combination of theoretical calculations, experimental validation, and advanced characterization techniques, the research team has demonstrated the potential of a novel catalyst design to reduce the reliance on costly metals while improving performance. By overcoming the challenges of scalability and powder consistency, this innovative catalyst brings us closer to realizing the vision of a hydrogen-based energy economy that is environmentally friendly and economically viable.

Leave a Reply