In a groundbreaking study conducted by researchers from Pohang University of Science and Technology (POSTECH), the Korea Research Institute of Chemical Technology, and Chonnam National University, a new technique for separating well-mixed mixtures has been developed. Led by Professor Jee-hoon Han, the team created a technology for synthesizing and purifying ionic liquids efficiently, a process that has the potential to revolutionize various industrial applications.
Ionic liquids are salts that remain in a liquid state at room temperature due to strong electrical interactions between their ions. These liquids possess unique properties such as nonflammability, low volatility, and thermal and chemical stability, making them valuable for industries like catalysis and electrolyte production. One of the most well-known ionic liquids is [bmim][BF4], prized for its stability and low toxicity.
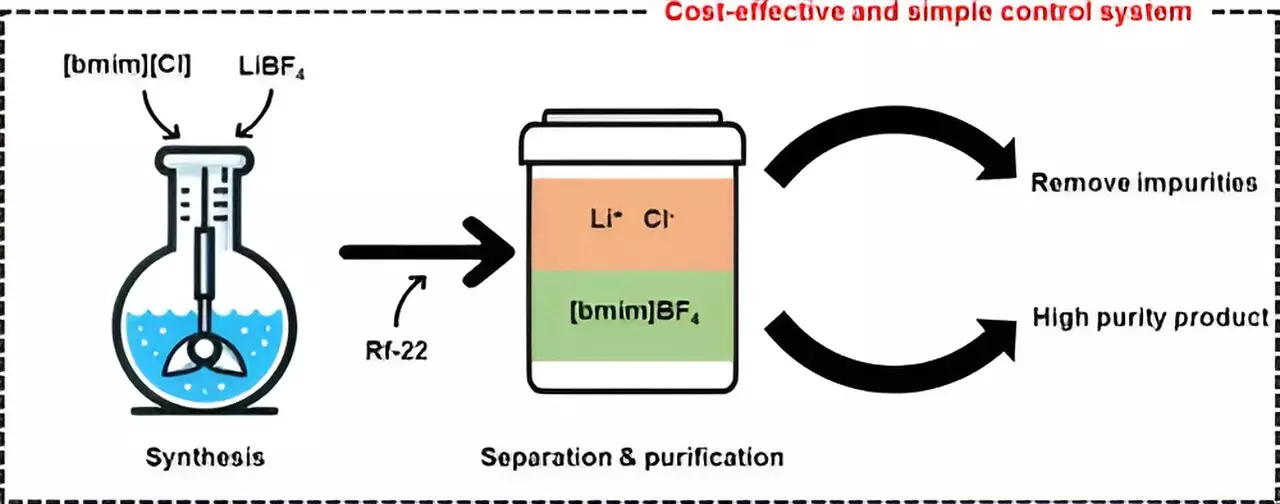
Traditionally, the synthesis of ionic liquids like [bmim][BF4] has been hindered by the complex and costly process of removing impurities such as lithium chloride (LiCl). However, the research team utilized halocarbon refrigerants, specifically chlorodifluoromethane (Rf-22), to synthesize [bmim][BF4] more economically and efficiently than previous methods. By using Rf-22 as a phase separation mediator, they were able to induce the separation of the mixture containing methylimidazole into two distinct layers, similar to the separation of oil and water.
Through a careful analysis of the ratios of [bmim][BF4], water, and halocarbon mixtures, the researchers were able to observe phase separation and apply their findings to a ternary phase diagram model. This model visually represents the composition and phases of a mixture and allows for predictions based on ingredient proportions. Using this modeling approach, the team successfully produced high-purity [bmim][BF4] with a purity exceeding 99%.
In addition to achieving high-purity ionic liquids, the researchers were able to recover and recycle the layer containing methylimidazole, further enhancing the economic feasibility of the purification process. Through process simulations, they determined that the minimum selling price for producing 1 ton of [bmim][BF4] per day would be approximately $12,000 per ton, making this technology more competitive than existing methods and showcasing its potential for commercialization.
The development of this new synthesis and purification technique for ionic liquids represents a significant advancement in the field of chemical engineering. By enhancing the efficiency and economic feasibility of producing high-purity ionic liquids like [bmim][BF4], the research team has opened up new possibilities for industrial applications and commercialization. This breakthrough marks a promising future for the utilization of ionic liquids in various industries.

Leave a Reply