The realm of neurological treatments is on the verge of a transformative breakthrough, thanks to advancements in transcranial focused ultrasound (tFUS). This innovative non-invasive technology employs high-frequency sound waves to target specific brain regions, raising hopes for effective therapies against chronic conditions like drug-resistant epilepsy and other disorders characterized by recurrent tremors. Researchers working collaboratively between institutions such as Sungkyunkwan University (SKKU), the Institute for Basic Science (IBS), and the Korea Institute of Science and Technology have made significant strides in refining this technology, enhancing its efficacy and application.
Despite the promise of previous brain sensors designed to interact with the cortical surface, notable limitations have hampered their clinical effectiveness. Traditional sensors encountered significant difficulties in conforming to the intricate anatomical folds of the brain, which adversely affected their ability to measure neural signals accurately. A leading team comprising Professors John A. Rogers and Dae-Hyeong Kim developed sensors that minimized some of these complications due to their ultra-thin designs. However, these sensors still struggled with adhering to highly curved brain regions, thus significantly affecting their functionality over extended periods.
Donghee Son, the lead author of the recent study published in *Nature Electronics*, highlighted that fluctuations in brain movement and cerebrospinal fluid flow could result in sensors detaching from their intended locations, leading to interruptions in data collection. This inconsistency is a serious barrier to achieving accurate long-term monitoring, raising questions about the reliability of existing treatments based on these technologies.
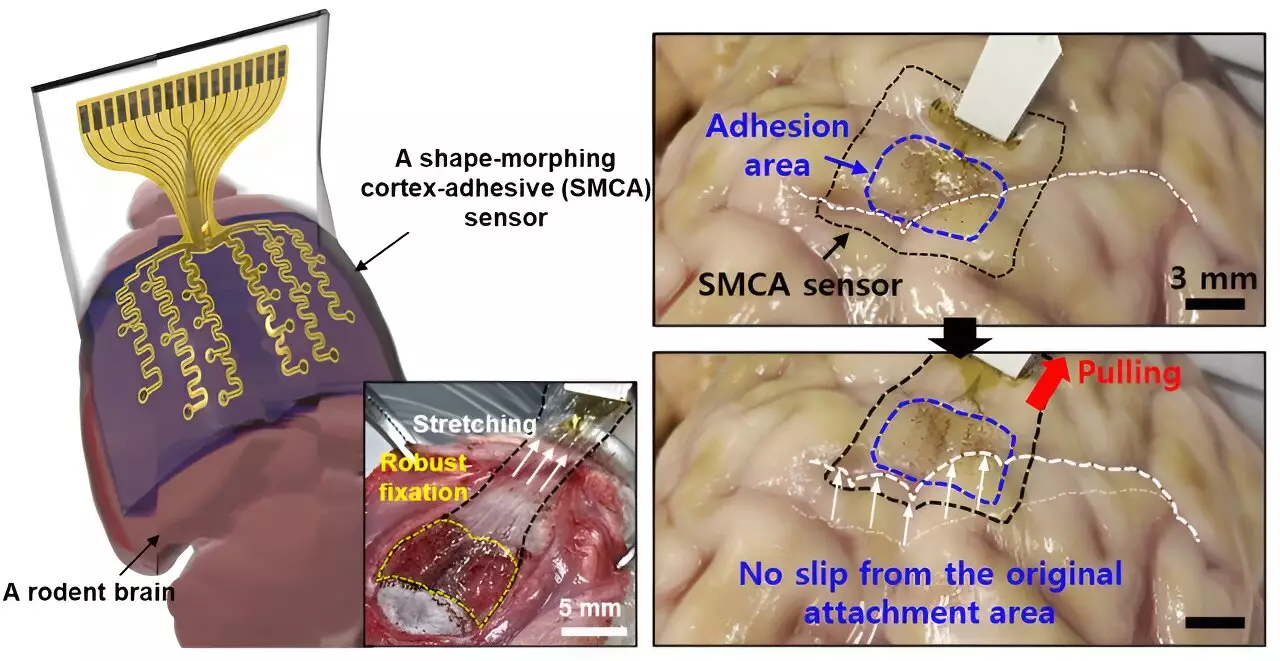
In response to these challenges, Son and his team embarked on developing an advanced sensor designed specifically to enhance adherence to the brain’s complex surfaces. Dubbed the ECoG, this revolutionary sensor boasts a unique multi-layer architecture that allows it to adapt dynamically to the highly variable contours of brain tissue. The functionality of the ECoG hinges upon three crucial layers: a hydrogel-based layer for strong tissue bonding, a self-healing polymer layer that conforms to anatomical variations, and an ultrathin stretchable layer outfitted with gold electrodes.
One of the ECoG’s most significant advantages lies in its ability to minimize noise from external mechanical movements, given its secure attachment to the brain. This characteristic addresses the critical problem of signal interference in conventional sensors, thereby enhancing the efficacy of treatments such as low-intensity focused ultrasound (LIFU) for epilepsy management. With the ECoG’s deployment, clinicians can potentially decouple patient-specific variances and optimize treatment protocols tailored to individual needs.
The importance of real-time brain wave monitoring cannot be overstated, particularly for customizing treatment strategies. Traditional sensors suffered from substantial noise distortion during ultrasound simulations, complicating the tracking of brain waves. However, the advancements represented by the ECoG sensor enable clearer visibility into patient brain activity. By drastically reducing noise interference, this technology holds promise for the foundation of personalized treatment plans in both epilepsy and various other neurological disorders.
The ability of the new sensor to bond with brain tissue and reduce vibrational artifacts represents a significant leap forward. By facilitating accurate measurements while simultaneously providing stimulation, the ECoG could potentially unlock new doors for tailored therapies that fit individual patient profiles, enhancing treatment responsiveness and efficacy.
Initial tests on living, awake rodents have yielded encouraging results, demonstrating the ECoG’s capability to measure brain wave activity and control seizure episodes effectively. As the research continues, the team envisions scaling up the sensor’s capabilities, with ambitions to create high-density arrays featuring an expanded number of electrode channels. The foresight surrounding such advancements includes broad applications—not just in the realm of epilepsy treatment, but also in diagnosing a diverse array of neurological conditions.
Ultimately, the primary objective revolves around designing a flexible and responsive sensor that integrates both tissue adhesion and shape-morphing technologies. This dual functionality not only enhances the precision of brain signal mapping but also opens new avenues for therapeutic advancements. With clinical trials on the horizon, the successful deployment of ECoG sensors could transform the landscape of neurological treatment by delivering improved diagnostic capabilities and more effective treatment methodologies for myriad brain disorders.
As science continues to refine the tools available for understanding and treating complex brain conditions, technologies like the ECoG sensor will stand at the forefront of innovation. Through enhanced signal accuracy and patient-centric treatments, transcranial focused ultrasound may soon become a mainstay in the therapeutic arsenal against epilepsy and beyond. The journey from research to clinical application promises to be as groundbreaking as the discoveries themselves, highlighting a future where precision medicine meets cutting-edge technology for the betterment of patient care.

Leave a Reply