California is rapidly transitioning to renewable fuels, but the state faces challenges in storing power for the electric grid. Solar power drops at night and declines in winter, while wind power ebbs and flows. As a result, natural gas is heavily relied upon to smooth out the highs and lows of renewable power generation. However, researchers at Stanford University, led by Robert Waymouth, are exploring a new technology for energy storage called liquid organic hydrogen carriers (LOHCs). This technology has the potential to revolutionize how renewable energy is stored and utilized.
One of the main challenges with renewable energy sources is the inability to store excess energy for times when it is needed. Batteries, such as lithium-ion technologies used in grid storage, smartphones, and electric vehicles, have limitations in terms of scale. This has led researchers to search for alternative systems that can supplement existing technologies. LOHCs have emerged as a promising candidate for storing and releasing hydrogen using catalysts and elevated temperatures, essentially functioning as “liquid batteries” that can store energy and efficiently return it as usable fuel or electricity.
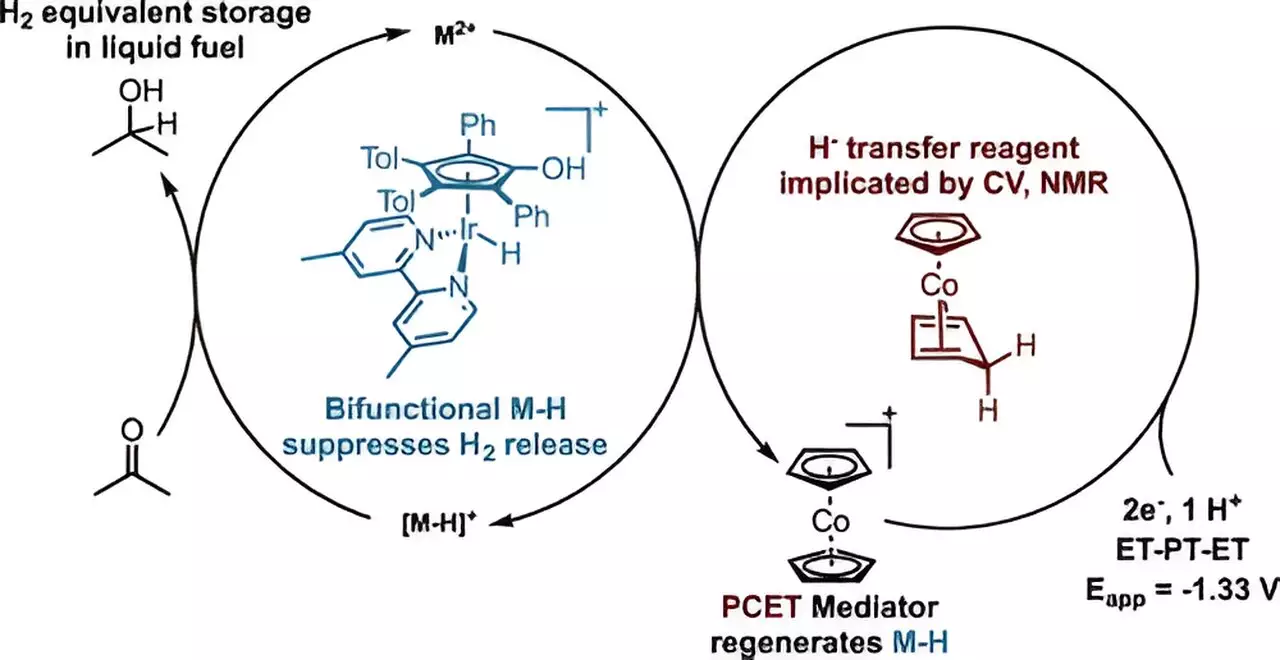
The Stanford research team is focusing on isopropanol and acetone as key ingredients in hydrogen energy storage and release systems. Isopropanol, also known as rubbing alcohol, is a high-density liquid form of hydrogen that can be stored or transported through existing infrastructure. When needed, it can be converted into a fuel for fuel cells or released as hydrogen for use without emitting carbon dioxide. However, current methods of producing isopropanol with electricity are inefficient, requiring a way to produce it directly from protons and electrons without generating hydrogen gas.
Daniel Marron, lead author of the Stanford study, identified a way to address this issue by developing a catalyst system that combines protons and electrons with acetone to selectively generate isopropanol without producing hydrogen gas. By using iridium as the catalyst and cobaltocene as a co-catalyst, the researchers were able to efficiently deliver protons and electrons to the iridium catalyst, enabling the direct conversion of acetone into isopropanol. This breakthrough not only paves the way for more affordable and scalable LOHC systems but also opens up possibilities for developing other catalysts using common materials like cobalt.
As the research on LOHCs continues to evolve, the hope is that these systems could significantly improve energy storage for various industries, energy sectors, and individual solar or wind farms. The elegant process of storing excess energy as isopropanol and converting it back into electricity when needed offers a promising solution to the challenges of renewable energy storage. With ongoing efforts to optimize catalyst systems and explore alternative materials, LOHCs have the potential to play a vital role in the future of renewable energy storage.
The development of liquid organic hydrogen carriers represents a significant advancement in energy storage technologies. By leveraging the unique properties of isopropanol and acetone, along with innovative catalyst systems, researchers are paving the way for a more sustainable and efficient energy future. As the demand for renewable energy continues to grow, the importance of effective energy storage solutions like LOHCs cannot be overstated. With further research and development, these systems could revolutionize the way we store and utilize renewable energy, contributing to a more sustainable and resilient energy grid.

Leave a Reply