The intersection of DNA and protein science represents a pivotal frontier in biomedical research, allowing scientists to leverage the unique properties of both to design innovative therapeutic agents. Recent breakthroughs have illuminated a pathway towards creating biohybrid molecules – entities that marry the specificity of nucleic acids with the diverse functionality of proteins. By tapping into the natural capabilities of bacteria, this approach promises an efficient means of producing a library of DNA-protein hybrids with potential applications in treating a multitude of diseases.
The traditional methodology for synthesizing biohybrid molecules has been a labor-intensive and often slow process, reliant on complex chemical techniques. The seminal work by researchers at the University of Illinois Urbana-Champaign, led by Professor Satish Nair and postdoctoral researcher Zeng-Fei Pei, marks a significant advancement in this field. Their research presents a compelling alternative: a streamlined method of harnessing bacterial enzyme systems that can synthesize hybrid molecules with remarkable efficiency.
Historically, the synthesis of biohybrids has involved extensive chemical manipulations, which not only make the process time-consuming but also limit scaling. In this new study published in *Nature Chemical Biology*, Nair and his team’s approach involves the exploitation of naturally occurring bacterial enzymes to produce these hybrids en masse. Rather than chemically synthesizing each molecule individually, they have established a method that could generate millions of compounds in a fraction of the time.
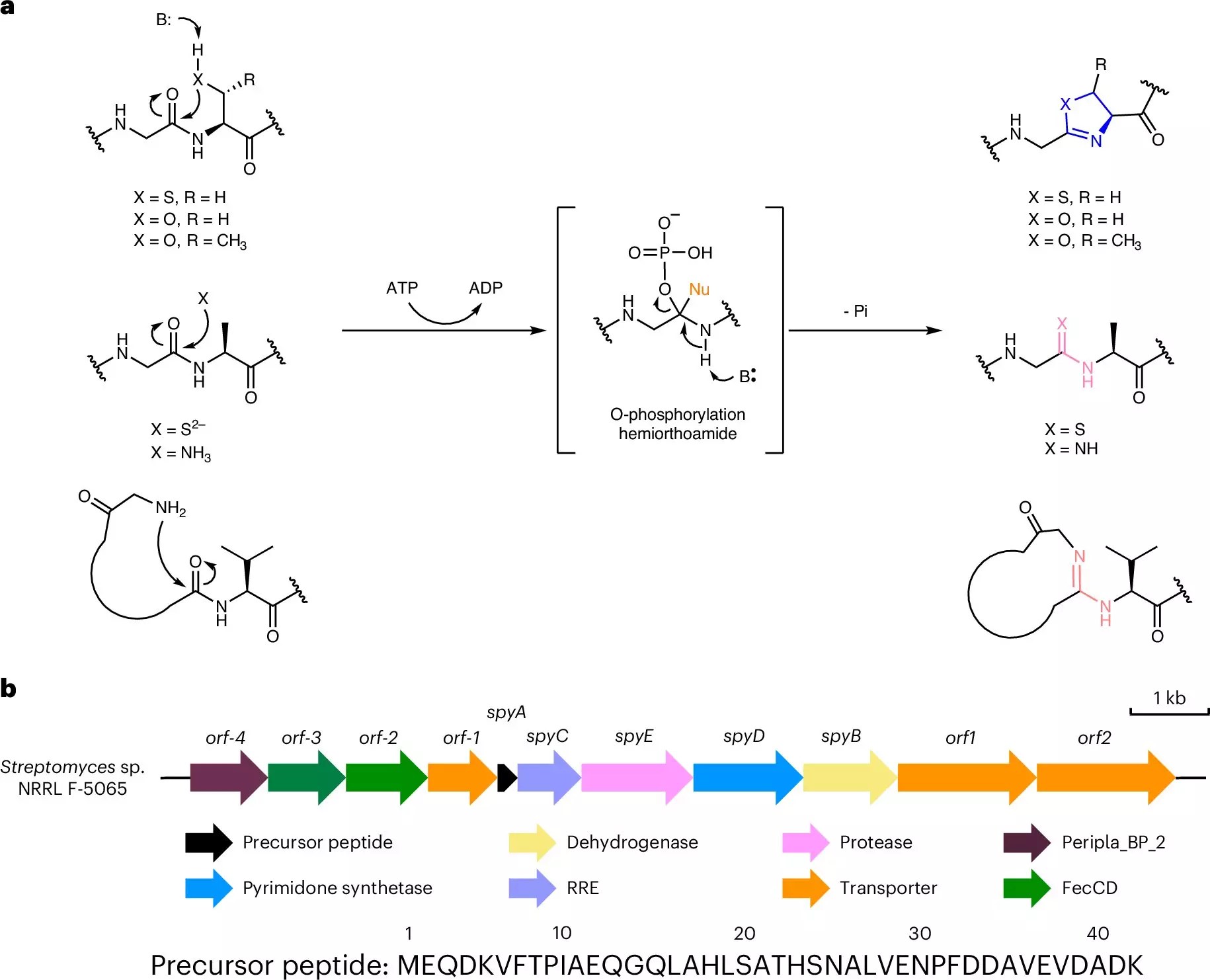
At the core of this revolutionary technique lie two bacterial enzymes that play complementary roles in the hybridization process. The first enzyme, YcaO, facilitates the conversion of specific amino acids within peptides into a structure that resembles the nucleobases found in DNA and RNA. This transformation is crucial as it lays the foundation for creating a binding site capable of facilitating interactions with nucleic acids.
The second enzyme employed in this process is a protease. Its role is to cleave the modified peptide at a specific point, yielding a fully functional nucleobase-protein hybrid. This two-step enzymatic reaction can be executed with only three initial components: the peptide and the two enzymes, significantly simplifying the process. Furthermore, the fact that this conversion can also occur within *E. coli* illustrates the potential for scaling up in laboratory settings.
Biohybrid molecules have unprecedented potential for therapeutic applications. The ability to design drugs that can selectively target mutated genes or noncoding RNA which contribute to pathological conditions could lead to revolutionary treatments for various diseases. The precision with which these hybrids can interrupt disease mechanisms may not only enhance therapeutic efficacy but also reduce off-target effects that plague many existing treatment modalities.
As Professor Nair noted, the integration of DNA’s specificity with the functional diversity of proteins marks a new chapter in drug development. Such integration could pave the way for creating “precision drugs” — therapeutics that act on exact molecular targets with the precision necessary to modulate biological processes without undesired side effects.
This discovery of the DNA-protein hybrid and the subsequent unraveling of its synthesis was not achieved in isolation. Collaboration between Nair’s team and researchers from the John Innes Centre marked a significant turning point. Their joint efforts underscore the importance of interdisciplinary partnerships in scientific discovery, allowing for a deeper understanding of molecular mechanisms.
Looking ahead, further exploration of these bacterial enzymatic pathways may reveal additional applications not just in therapeutics, but in diagnostics and biotechnology. The prospect of utilizing bacteria as biocatalysts for rapid, large-scale production of biohybrids opens a new realm for research, potentially leading to ready access to novel biomolecules for research labs worldwide.
The landscape of therapeutic development is on the verge of transformation, thanks to advancements in the integration of DNA and protein functions through novel biohybrid molecules. The capacity for high-throughput, efficient production of these hybrids heralds a new era in precision medicine. As researchers continue to navigate this intricate molecular terrain, the potential for addressing complex diseases with tailored therapeutic strategies becomes ever more tangible. The exciting implications of this work could soon lead to breakthroughs that reshape our understanding and treatment of disease.

Leave a Reply