In a groundbreaking study, scientists have discovered a previously unidentified mechanism that drives self-organization in active matter integral to bacterial cell division. This exploration, carried out by Anđela Šarić and her team at the Institute of Science and Technology Austria (ISTA), has broad implications, particularly in the field of synthetic materials and self-healing systems. Published in Nature Physics, this work illuminates how seemingly lifeless cellular components can orchestrate complex formations reminiscent of living structures.
As researchers strive to comprehend the enigma of self-organization—an indelible feature of life—questions arise about how non-living molecules coordinate their assembly, make critical decisions about disassembly, and align themselves into functional structures without an external directive.
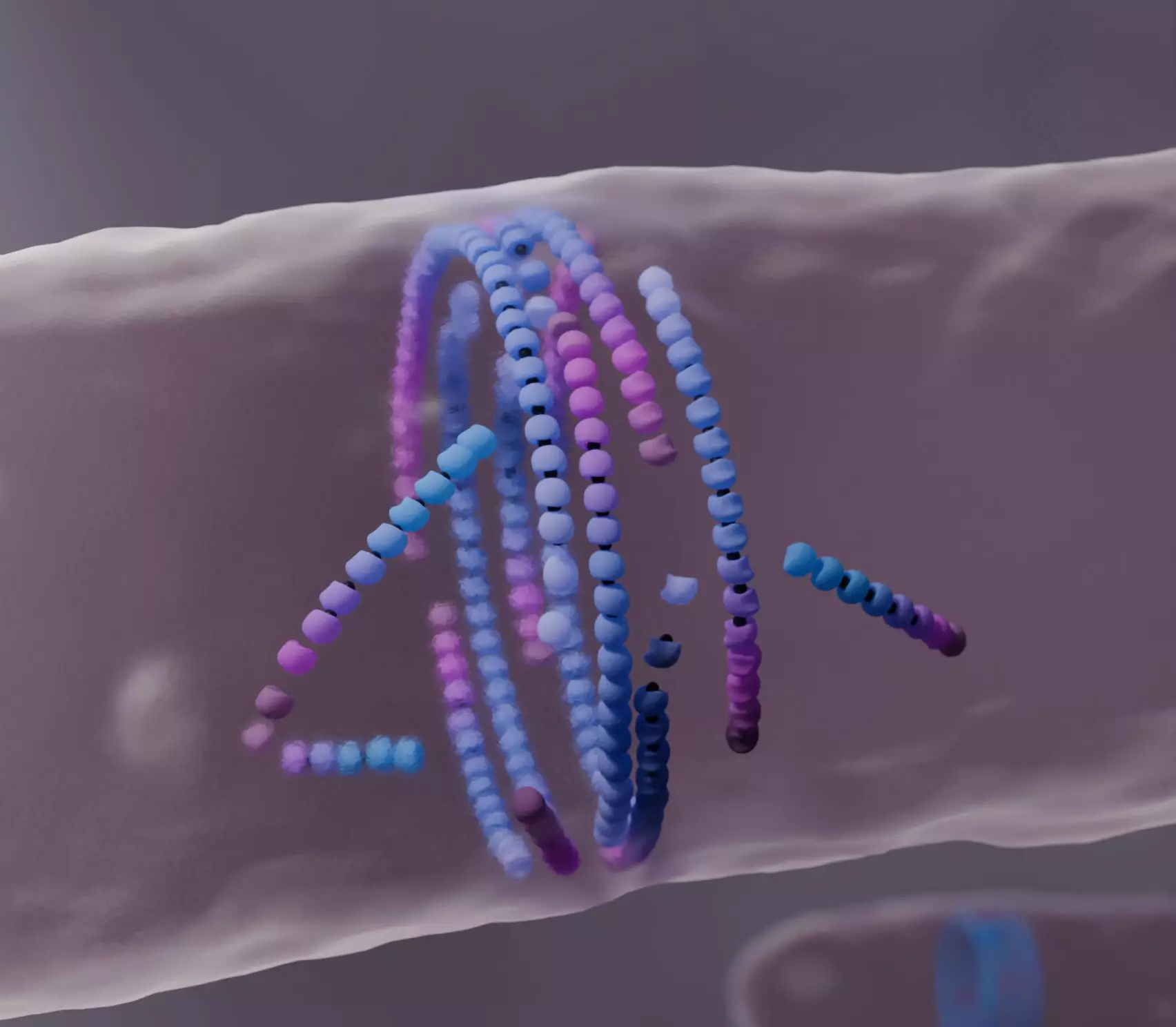
Research led by Professor Šarić and Ph.D. student Christian Vanhille Campos takes a deep dive into the dynamics of a critical protein, FtsZ. This protein plays a pivotal role in bacterial cell division by forming a ring structure—often referred to as the bacterial division ring—at the center of the cell as it prepares to divide. The division ring is crucial for building the new cell wall that separates the two daughter cells. Despite its significance, the exact mechanics of FtsZ’s self-assembly have remained enigmatic until now.
Using sophisticated computational modeling, the researchers aimed to uncover the factors influencing the self-assembly of FtsZ. Their findings reveal an intricate interaction between misaligned FtsZ filaments and their environment—specifically how these filaments “die” upon colliding with obstacles. Rather than pushing through, these misaligned structures undergo a form of breakdown, allowing for the realignment and reassembly into the functional bacterial division ring. This extraordinary process emphasizes the notion that in self-organizing systems, failure can often facilitate broader structural success.
Historically, models of active matter focused on self-propulsion, indicating the forward momentum of filaments as they assemble. However, these conventional frameworks tended to overlook the continuous turnover of filament subunits and often exaggerated the forces involved in assembled structures. The Šarić group’s work challenges this perspective by proposing that the essence of active matter lies not in relentless motion but in the dynamic interplay of creation and decay—essentially a cycle of life perpetuated through molecular turnover rather than sheer force.
Vanhille Campos notes that this new understanding reshapes how scientists view active matter. Instead of a linear trajectory towards assembly, the interactions of these filaments reflect a complex dance characterized by adaptability and responsiveness to the surrounding environment.
The researchers’ findings find validation through collaborative efforts with experimentalists from other institutes, particularly at The University of Warwick and ISTA. Their empirical observations of FtsZ in live bacterial cells corroborate the computational model, revealing that the birth and death of FtsZ filaments are indeed fundamental to forming the division ring. This synergy provides a perfect example of how interdisciplinary collaboration can yield robust scientific insights that might remain hidden within isolated research endeavors.
In meetings like the “Physics Meets Biology” conference, the exchange of ideas and results between groups paved the way for new understandings. The alignment of experimental observations with computational predictions not only builds confidence in the model but also stimulates new questions regarding the implications of these findings in broader biological and material sciences.
One of the most exciting applications of this research lies in the potential development of synthetic self-healing materials. If living systems can self-organize and heal through molecular turnover, how can scientists replicate these processes in non-living materials? The findings suggest a pathway toward creating synthetic systems that exhibit similar dynamic properties, leading to innovations that would have previously seemed like science fiction.
Šarić herself is inspired by the potential applications of creating “living” matter from inanimate substances. As her team advances their studies, they aim to further explore how the FtsZ division ring facilitates the construction of new cellular walls, a process that could inform the design of smart materials capable of self-repair.
This study does not just advance our understanding of bacterial life; it opens doors to reevaluating our conceptual frameworks of self-organization and life’s fundamental processes. As we learn more about how active matter operates, we inch closer to answering profound questions about life’s origins and the mechanisms that allow the inanimate to bear life-like qualities. Ultimately, the intersection of biology and materials science illuminated by this research underscores a future where the marvels of nature inspire the innovations of tomorrow.

Leave a Reply