The process of photocatalytic hydrogen evolution from water is crucial for sustainable hydrogen production. However, there is a lack of understanding regarding the direct influence of the microscopic structure of interfacial water molecules on the reactivity of photocatalysis. A recent study published in the Journal of the American Chemical Society delved into this aspect, shedding light on the roles of interfacial hydrogen bond structure, dynamics, and the optimal environment for promoting H2 evolution.
Researchers led by Toshiki Sugimoto, Associate Professor at the Institute for Molecular Science, conducted a comprehensive investigation into the impact of interfacial hydrogen bond networks using various TiO2 photocatalysts. By controlling the thickness of adsorbed water layers from sub-monolayer to multilayers, they were able to establish a direct correlation between H2 formation rate and the microscopic structure of hydrogen bond networks.
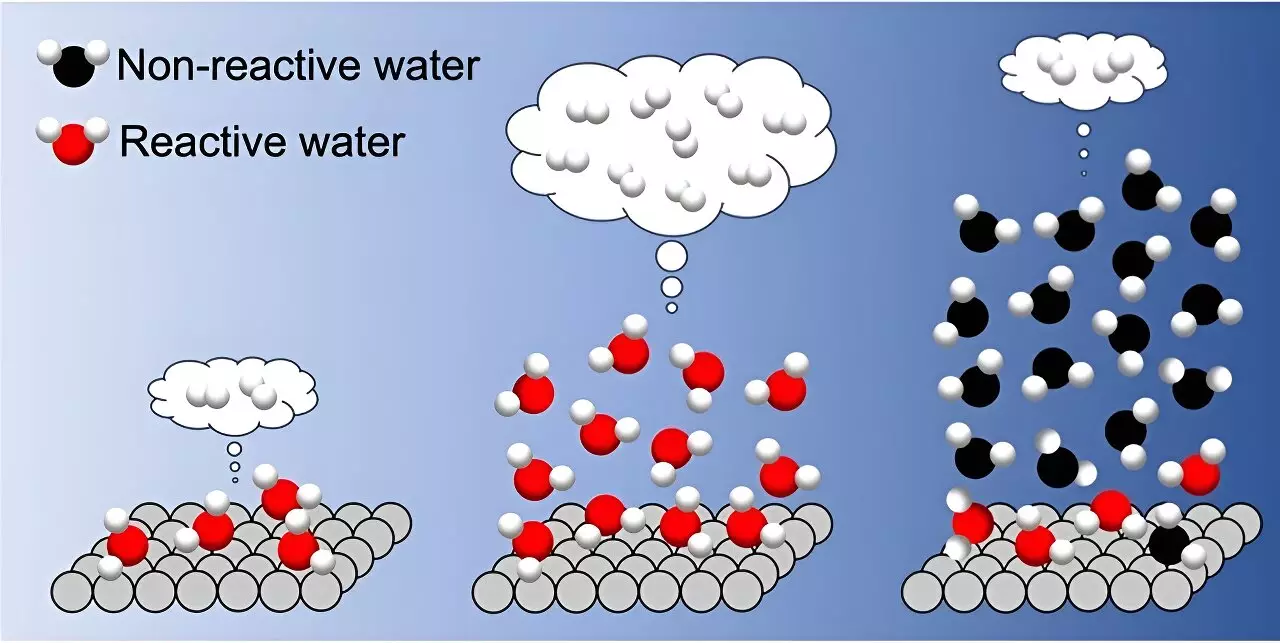
The study revealed that the presence of reactive water molecules extended beyond the first adsorbed layer, with an increase in H2 formation rate observed up to three layers of water adsorption. However, a dramatic decrease in H2 formation rate was noted when more than three layers of water covered the TiO2 surface. This decrease was attributed to the strengthening of interfacial hydrogen bonds due to many-body interactions among adsorbed water molecules.
Optimizing Photocatalytic Efficiency
Based on their microscopic insights, the researchers proposed that depositing three water layers in a water vapor environment is optimal for enhancing photocatalytic hydrogen evolution. This finding challenges the traditional approach of studying photocatalysis in aqueous solution environments and opens up new possibilities for designing innovative photocatalytic systems for sustainable energy production.
The discoveries made in this study have significant implications for the future of renewable energy production. By understanding the critical role of interfacial water molecules and their hydrogen bond networks, researchers can now design more efficient photocatalysts for next-generation energy solutions. The shift from liquid-phase reaction systems to water vapor environments represents a paradigm shift in the field of photocatalysis and paves the way for the development of novel and sustainable energy technologies.
The study on the impact of interfacial water molecules on photocatalytic hydrogen evolution provides valuable insights into the molecular-level design of efficient photocatalytic systems. By uncovering the crucial roles played by interfacial hydrogen bond structure and dynamics, researchers can now work towards optimizing the performance of photocatalysts for sustainable hydrogen production. This research opens up new avenues for the development of innovative energy solutions and underscores the importance of understanding the microscopic properties of interfacial water molecules in photocatalysis.

Leave a Reply