Esters, the compounds responsible for the sweet smell of fruits like strawberries, are widely used in various industries for producing chemicals, pharmaceuticals, and cosmetics. However, the traditional methods of breaking down esters can be costly both financially and environmentally. A team of researchers from the National Institutes of Natural Sciences (NINS) in Japan has introduced a groundbreaking approach using light as an energy source to reduce esters and create valuable alcohols.
Breaking down esters involves adding electrons to the compound, which forces the ester groups to reduce into basic components. The conventional methods require the use of highly reactive and hard to handle metal reductants. The researchers at NINS are exploring the use of sustainable photocatalysts that can activate when excited by light and facilitate the electron transfer process without the need for reactive metal reductants.
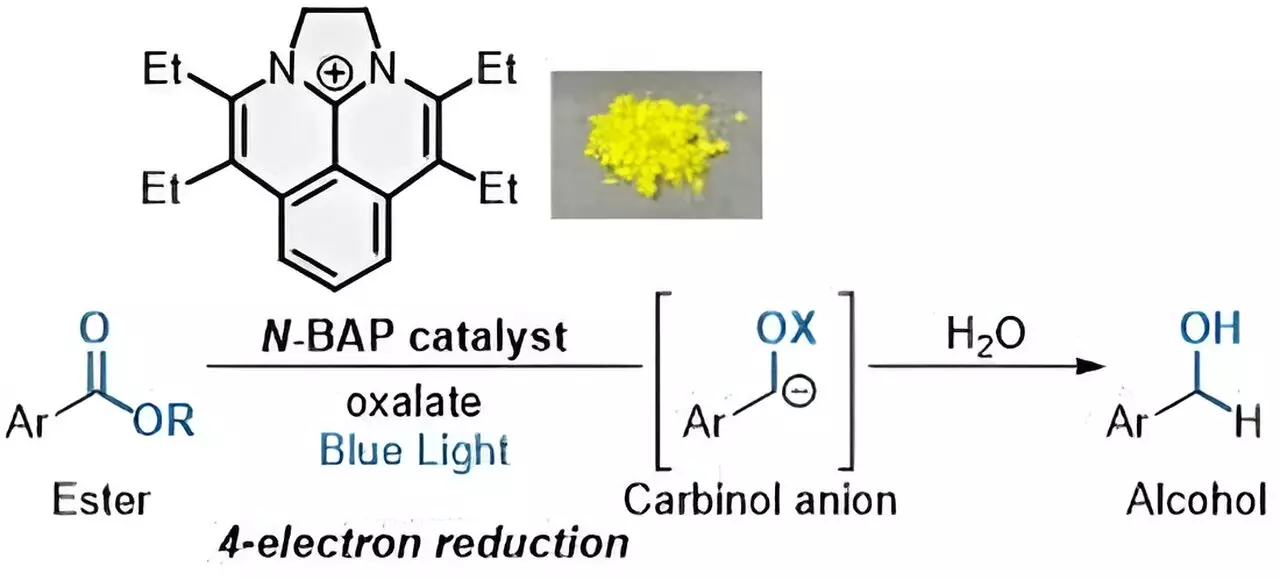
The newly developed photocatalyst named “N-BAP” allows for a sequential four-electron reduction of esters to produce alcohols. When irradiated with blue light, the N-BAP catalyst initiates a reaction that combines with water and a carbon-based chemical group. By introducing oxalate as a traceless reductant, the reaction can efficiently add four electrons in quick succession, resulting in the desired alcohols.
Photocatalytic reactions using visible light as an energy source have gained significant attention as sustainable methods aligned with the United Nations’ Sustainable Development Goals (SDGs). Unlike traditional methods, photocatalytic reduction eliminates the need for expensive and non-renewable noble metals and reduces the use of limited organic compounds. The innovative approach developed by the researchers at NINS opens up new possibilities for efficient and environmentally friendly ester reduction processes.
The ability to efficiently reduce esters to form alcohols through a quadruple electron transfer process has significant implications for various industries. The use of sustainable photocatalysts and light energy can revolutionize the way chemical compounds are synthesized, leading to greener and more cost-effective production methods. The researchers are optimistic about the potential applications of their novel approach in pharmaceuticals, cosmetics, and other industrial sectors.
The innovative use of light as an energy source for ester reduction represents a major milestone in the field of organic synthesis. The development of the N-BAP photocatalyst and the sequential four-electron reduction process demonstrate the potential for sustainable and efficient chemical reactions. By leveraging the power of light, researchers are paving the way for a more environmentally friendly and economically viable approach to ester reduction and alcohol production.

Leave a Reply