Peptides have been gaining attention in the pharmaceutical industry as valuable therapeutic agents due to their ability to target complex biological processes with more precision compared to small-molecule drugs. They are also less complex and more cost-effective than large biological drugs like antibodies. The market has seen the emergence of over 100 FDA-approved peptide drugs, with around 40 of them containing tryptophan (Trp) residues, a key amino acid that plays a crucial role in drug-target interactions.
While modifying Trp residues in peptides can enhance drug properties such as stability and bioavailability, it poses challenges due to the high level of selectivity required for effective transformations. Peptides’ nucleophilic nature also makes them sensitive to redox conditions, adding complexity to the modification process. Another obstacle is the limited solvents available for dissolving unprotected peptides, making site-specific late-stage peptide modifications a daunting task for researchers.
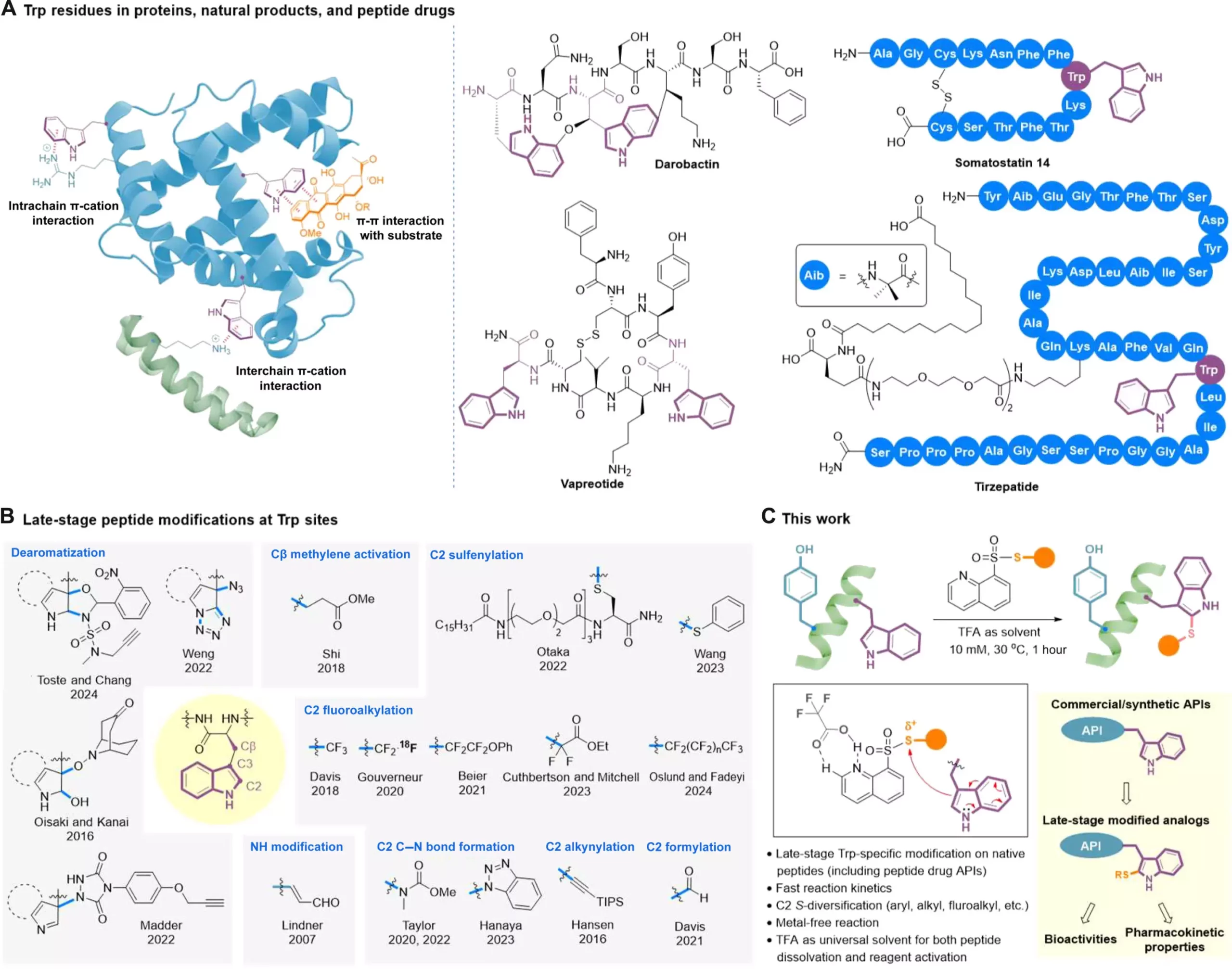
A recent study led by Professor Xuechen Li from The University of Hong Kong introduced a clickable tryptophan modification strategy that allows for late-stage diversification of peptides. This innovative approach involves a catalyst-free C2-sulfenylation reaction using S-modified quinoline-containing thiosulfonate reagents. By utilizing this method, researchers were able to efficiently introduce various functional groups onto Trp residues within native peptide structures, including trifluoromethylthio, difluoromethylthio, and alkylthio.
The study successfully applied the late-stage modification technique to well-known peptide drugs such as somatostatin and daptomycin, showcasing its versatility in modifying a range of peptide-based pharmaceuticals. The enhanced bioactivity and serum stability observed in modified melittin analogs further underscore the potential of this method in drug development. Additionally, the method’s applicability to natural products and drug leads from various screening methods highlights its usefulness in creating molecular libraries and functional probes.
Future Implications
The single-step clickable late-stage Trp modification method developed by Professor Li’s team is poised to become a valuable tool for medicinal chemists, peptide chemists, and chemical biologists in optimizing drug activities and pharmacokinetic properties. Its cost-efficiency and ability to generate structural analogs make it a promising platform for advancing drug development efforts and meeting the evolving needs of the pharmaceutical industry.

Leave a Reply