The quest for sustainable energy alternatives is paramount in addressing the climate crisis. Among the potential transformative solutions, seawater electrolysis stands out as a promising method for generating hydrogen, a clean energy carrier. Despite its potential, the direct use of seawater in electrolysis has been impeded by specific challenges. Notably, the corruption of anodes due to chloride ions and the high costs associated with effective catalysts have been significant roadblocks. Recent advancements in the development of bifunctional catalysts have paved the way for overcoming these hurdles, enabling a more straightforward approach to harnessing the power of seawater.
Nickel-iron (NiFe) catalysts have emerged as strong contenders due to their favorable external characteristics and economical production costs. These catalysts exhibit high intrinsic activity, which enhances their suitability for both hydrogen and oxygen evolution reactions. By focusing on self-supported NiFe materials, researchers have provided a solution that could revolutionize the efficiency of seawater electrolysis. Recent studies bring attention to the utilization of wood-based carbon (WC) structures as a foundation for these catalysts. The porous architecture and enhanced electrical conductivity of WC are key properties that facilitate better performance in electrochemical applications.
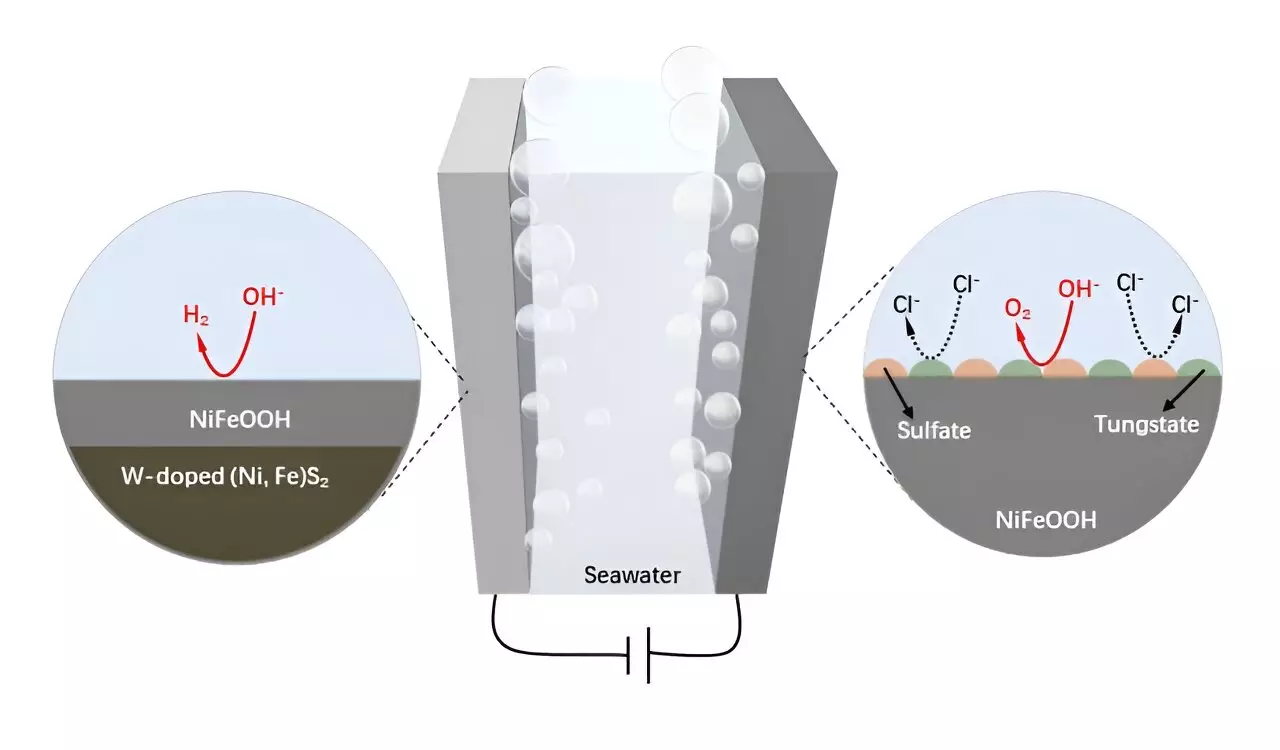
Recent research efforts led by Prof. Hong Chen and his colleagues have introduced innovative approaches to improve the stability and effectiveness of NiFe-based electrodes in seawater environments. The incorporation of tungsten (W) into the structure of NiFe was a crucial development that demonstrated enhanced resistance to corrosive elements in seawater. This method culminated in the creation of a W-doped NiFe sulfide (W-NiFeS) supported on WC, designed explicitly for efficient seawater electrolysis. The unique structural features of this electrode, characterized by intricate microchannels and a high degree of porosity, signify a substantial leap forward in electrochemical design.
The resultant W-NiFeS/WC electrode exhibited remarkable performance metrics that outstrip traditional catalysts in both the oxygen evolution and hydrogen evolution reactions. Its outstanding electrocatalytic properties are attributed to the lush redox-active centers within its composition, which allow for effective charge transfer and enhanced stability in alkaline seawater. Observations from laboratory tests highlight a noteworthy transformation during operation, where a self-generated layer of anti-corrosive compounds arises, thereby fortifying the electrode’s durability and efficacy.
Beyond its technical merits, this research signifies a paradigm shift towards sustainable practices by utilizing wood waste in the design of advanced electrochemical devices. Transforming abundant wood waste into valuable catalysts demonstrates a commitment to the principles of a circular economy — minimizing waste while promoting eco-friendly hydrogen production. As the world pivots towards sustainability, the successful application of these innovative catalysts in seawater electrolysis can symbolize a major advancement in achieving green hydrogen production on a global scale.
Self-supported W-doped NiFe catalysts represent a vital innovation in the realm of seawater electrolysis. With their impressive performance and cost-effective nature, they pave the way for more efficient hydrogen production processes. As research continues to evolve in this field, the implications could resonate far beyond the energy sector, fostering advancements in sustainable technology and materials science that contribute to a greener future for all.

Leave a Reply