Tungsten pentaboride, also known as WB5-x, has recently gained attention from researchers for its potential as a catalyst in various industrial applications. A group of scientists led by Professor Alexander Kvashnin from Skoltech’s Energy Transition Center conducted a study to explore the unique properties of tungsten pentaboride and its effectiveness as a catalyst in different processes.
Stable Surfaces and Catalyst Properties
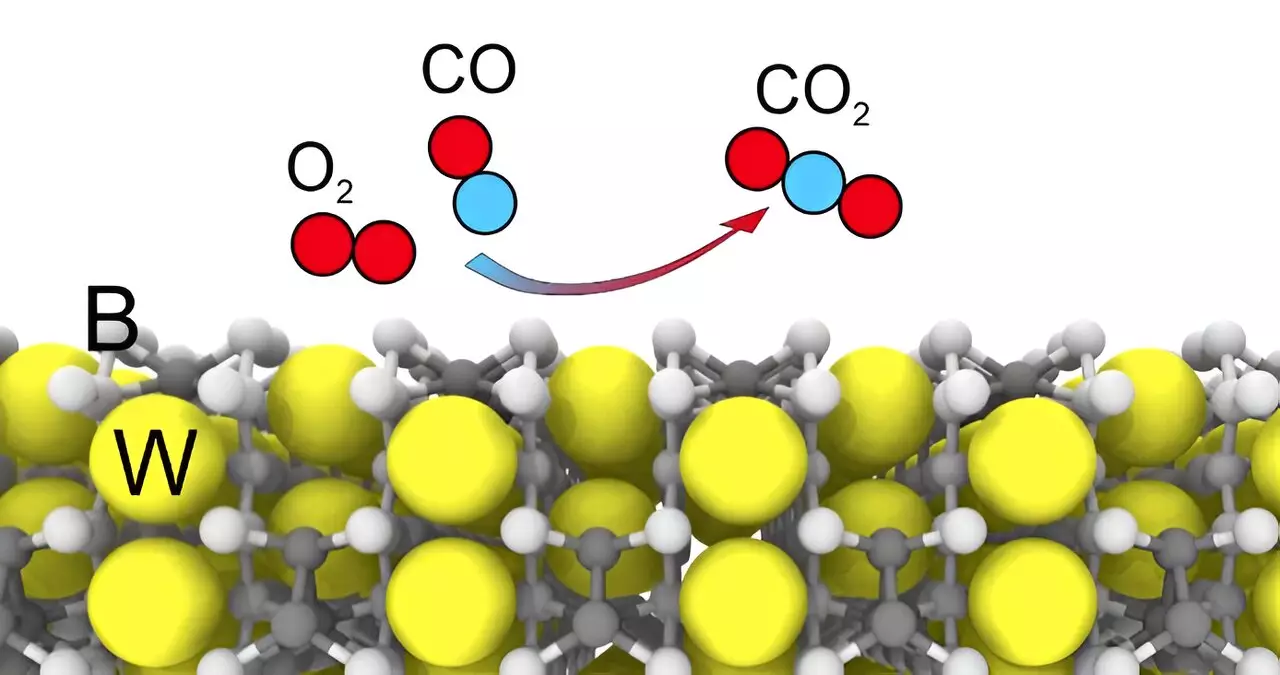
The researchers in the study identified stable surfaces of the WB5-x crystal and discovered that this new catalyst is not affected by sulfur-containing impurities, ensuring that it maintains its activity level. This finding is crucial as many traditional catalysts are prone to being poisoned by such impurities, leading to a decrease in efficiency over time. Tungsten pentaboride shows promise as a catalyst or co-catalyst in applications such as filtering industrial exhaust gases, extracting precious metals, producing hydrogen through photocatalysis, and other fields.
One of the key observations made in the study is the correlation between the amount of boron in the compound and its catalytic properties. Surprisingly, the researchers found that a higher concentration of boron in the compound leads to improved catalytic activity. This discovery challenges the conventional wisdom that active catalyst centers are typically metal atoms. The high boron content in tungsten pentaboride makes it a particularly promising catalyst, as it contains a significant amount of boron compared to other boron-metal compounds.
An important advantage of tungsten pentaboride identified in the study is its resistance to poisoning from sulfur-containing compounds. Sulfur is a common contaminant in many industrial processes, and it often leads to the deactivation of catalysts. Tungsten pentaboride’s ability to withstand poisoning from sulfur compounds makes it a valuable asset in catalytic applications where such contaminants are present. This resistance ensures that the catalyst remains active and maintains its efficiency over an extended period.
Implications for Industrial Applications
The researchers also examined the interactions of tungsten pentaboride with various gas molecules, including carbon dioxide, to assess its poisoning susceptibility. Preliminary data indicates that the catalyst is not affected by these molecules, highlighting its potential for long-term use in diverse industrial settings. This advantage, coupled with the relative affordability of tungsten pentaboride compared to catalysts based on noble and rare earth metals, makes it a highly attractive option for industrial applications requiring stable and efficient catalytic processes.
The study on tungsten pentaboride sheds light on its promising role as a catalyst in different industrial processes. By understanding its stable surfaces, enhanced catalytic properties with boron, resistance to poisoning, and implications for various applications, researchers have paved the way for the widespread adoption of tungsten pentaboride in catalytic processes. Further research and development in this area hold the potential for significant advancements in industrial catalysis and environmental technology.

Leave a Reply