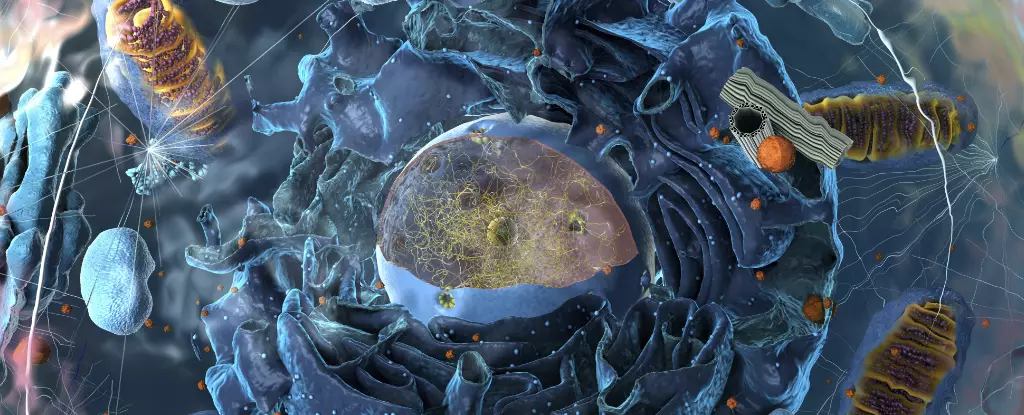
In the realm of cellular biology, the historic understanding of organelles has been foundational. High school students often learn about these membrane-bound units, such as mitochondria, lysosomes, and the nucleus, each serving specific and essential roles for life. However, the scientific landscape began to shift dramatically in the mid-2000s with the discovery of membraneless organelles, known as biomolecular condensates. This revelation has not only expanded our understanding of cellular organization but has also initiated a fundamental reassessment of the principles governing life itself.
Understanding Biomolecular Condensates
Biomolecular condensates challenge the traditional paradigm by demonstrating that cellular compartments do not always require a delineating membrane. These structures can be envisioned as dynamic entities resembling the blobs in a lava lamp. In this metaphor, clusters of proteins and RNA molecules coalesce into gel-like droplets, creating an environment that facilitates specific biochemical interactions. Unlike traditional organelles, which are compartmentalized and defined by membranes, biomolecular condensates form through molecular interactions that promote clustering based on compatibility among their components. This phenomenon raises intriguing questions about the underpinning mechanisms that drive cellular organization.
Research indicates that there are approximately thirty known types of biomolecular condensates—an impressive number considering there are only around a dozen recognized membrane-bound organelles. Among these membraneless structures are stress granules and ribosomes, which are critical for cellular function. Nevertheless, many condensates lack clearly defined roles, leading scientists to speculate about their diverse and potentially unexplored functionalities.
The emergence of biomolecular condensates has significant implications for protein chemistry. Traditionally, it was understood that a protein’s structure was intrinsically linked to its function—a principle that has guided biochemical research for decades. However, the discovery of intrinsically disordered proteins (IDPs) has illuminated an exception to this rule. These proteins, which do not possess a stable or defined structure, can still perform vital functions within cellular contexts.
The association between IDPs and biomolecular condensates reveals a new dimension of protein behavior, as many IDPs tend to aggregate into these unique structures. By studying the roles of these condensates, researchers have gained insights into the flexible and variable nature of protein behavior, challenging long-standing assumptions. This paradigm shift also generates new questions about the nature of these structures and their importance within cellular processes.
Implications for Prokaryotic Cells
Traditionally, prokaryotic cells—such as bacteria—were characterized as simple cellular entities lacking complex organelles. However, recent studies have disrupted this notion by identifying biomolecular condensates in bacterial cells. Interestingly, approximately 6% of bacterial proteins show disordered regions, a stark contrast to 30-40% observed in eukaryotic proteins. These findings suggest that bacterial cells possess a more intricate internal structure than previously assumed, including condensates that facilitate key cellular processes.
The understanding that prokaryotic cells may utilize biomolecular condensates to regulate functions such as RNA synthesis and degradation adds another layer of complexity to the model of cellular life. This revelation compels scientists to reconsider the evolutionary history of cellular organisms and the development of sophisticated life forms.
Insights into the Origins of Life
One of the remarkable implications of studying biomolecular condensates involves the origins of life on Earth. The RNA world hypothesis posits that early life forms were RNA strands capable of self-replication. Traditionally, this hypothesis relied on the assumption that such molecules required lipid membranes for protection and organization. However, the discovery that RNAs can spontaneously form aggregates through biomolecular condensation proliferates new possibilities for understanding life’s origins.
If RNA can create functional compartments without lipid membranes, it raises intriguing options for the existence of primordial life. This finding suggests that life may have emerged from more straightforward chemical processes than previously envisioned, emphasizing the significance of biomolecular condensates in the narrative of life’s beginnings on Earth.
The exploration of biomolecular condensates signifies a new frontier in biological research, extending to disease mechanisms and therapeutic interventions. For conditions like Alzheimer’s disease and other neurodegenerative disorders, understanding how condensates misbehave may provide insights into potential treatment strategies. Developing therapies that can modulate these condensates—either promoting their formation or encouraging their dissolution—holds promise for innovative medical applications.
As research progresses, it is likely that the intricate functions of individual biomolecular condensates will be elucidated, establishing a new lexicon for cell biology. Future high school biology curricula may need to adapt to this evolving landscape, preparing students for an enriched understanding of cellular biology that transcends traditional memorization of organelles.
The discovery and ongoing study of biomolecular condensates reveal a complex and dynamic world within cells, challenging long-held assumptions and providing new frameworks for understanding life at the molecular level. As biologists continue to unravel these mysteries, the implications for biology, disease, and the origins of life will undoubtedly expand, shaping our comprehension of life itself.

Leave a Reply