The waste-to-wealth movement has sparked a renewed interest in technologies that can convert greenhouse gases into valuable materials. Among these technologies, the catalytic conversion of methane into methanol has emerged as a promising solution. Methanol is a widely used industrial solvent and raw material for various chemical synthesis processes. However, the traditional industrial process for converting methane to methanol is known to be extremely energy and resource-intensive.
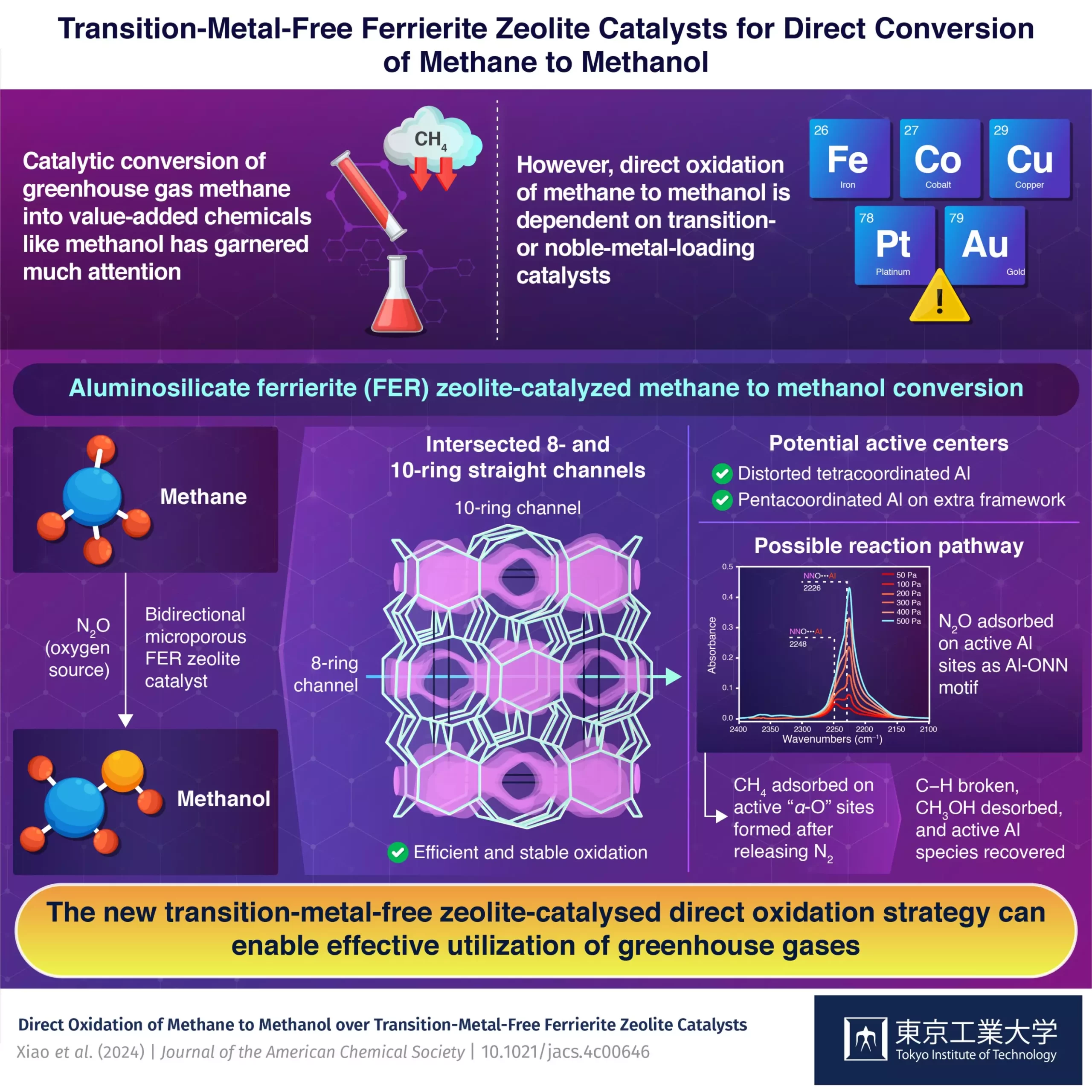
In a recent study conducted by a team of researchers from the Nanospace Catalysis Unit at the Institute of Innovative Research at Tokyo Institute of Technology, a groundbreaking solution to the existing challenges was discovered. This solution comes in the form of transition-metal-free aluminosilicate ferrierite (FER) zeolite. Unlike most catalyst systems, which rely on rare and expensive transition or noble metals, FER zeolite offers a unique alternative for the direct oxidation of methane to methanol.
Unlocking the Potential of FER Zeolite
The key to the success of this new catalytic system lies in the structural properties of FER zeolite. With 2-dimensional structure, 8-ring channels, and intersected 10-ring channels, FER zeolite is highly stable towards both chemical and thermal treatments. This stability allows for the efficient and effective conversion of methane to methanol. By utilizing nitrous oxide as the source of oxygen, the researchers were able to achieve the direct oxidation of methane to methanol for the first time.
Understanding the Mechanism of Action
To gain insight into the active sites within the zeolite catalyst, the research team employed Fourier transform infrared spectroscopy (FTIR) and magnetic resonance spectroscopy. Through these tests, they were able to identify distorted tetracoordinated Al in the framework and pentacoordinated Al in the extra framework as potential active centers. By studying the nitrous oxide adsorption FTIR spectra and observing reaction performances under different oxidants, the team was able to elucidate the reaction pathway.
The transition-metal-free zeolite-catalyzed direct oxidation process demonstrated impressive results, with a methanol production rate of 305 µmol g^-1 min^-1 and high selectivity for methanol and dimethyl ether. This performance surpassed many transition metal-loaded catalysts, showcasing the potential of FER zeolite in methane to methanol conversion. The researchers believe that their findings will pave the way for new advancements in the direct oxidation of methane to methanol using transition-metal-free aluminosilicate zeolites.
The innovative approach presented in this study holds great promise for addressing the challenges associated with methane to methanol conversion. By reducing greenhouse gas emissions and enabling the synthesis of valuable chemicals from unwanted raw materials, this research offers a sustainable and economically viable solution for the future. The utilization of transition-metal-free aluminosilicate zeolites marks a significant step towards a more environmentally friendly and efficient conversion process.

Leave a Reply