Cryo-electron tomography (cryo-ET) has emerged as a powerful tool for visualizing and analyzing cellular structures in their natural environment. A recent study conducted by researchers at the MPI of Biochemistry in Martinsried and the University Medical Center Göttingen utilized cryo-ET to investigate protein folding helpers, known as chaperonin complexes, in the bacterium E. coli. These chaperonins play a crucial role in assisting newly synthesized proteins to fold correctly into their functional form. The study delved into the folding reaction with unparalleled detail, tracking conformational changes in the chaperonin and its interactions with the client protein within the folding chambers, offering insights that have now been published in the prestigious journal Nature.
Proteins serve as the fundamental building blocks of life, requiring a specific three-dimensional structure to carry out diverse cellular functions. Chaperonins, such as those examined in this study, facilitate the folding process of proteins to ensure their proper functionality. F.-Ulrich Hartl, a renowned biochemist and Director at the MPI of Biochemistry, emphasizes the significance of chaperonins by stating that they are indispensable for cellular function and survival across various organisms. Furthermore, misfolded proteins have been associated with severe diseases like Alzheimer’s and Parkinson’s, underscoring the critical importance of understanding chaperonin structure and function in the development of therapeutic strategies for such conditions.
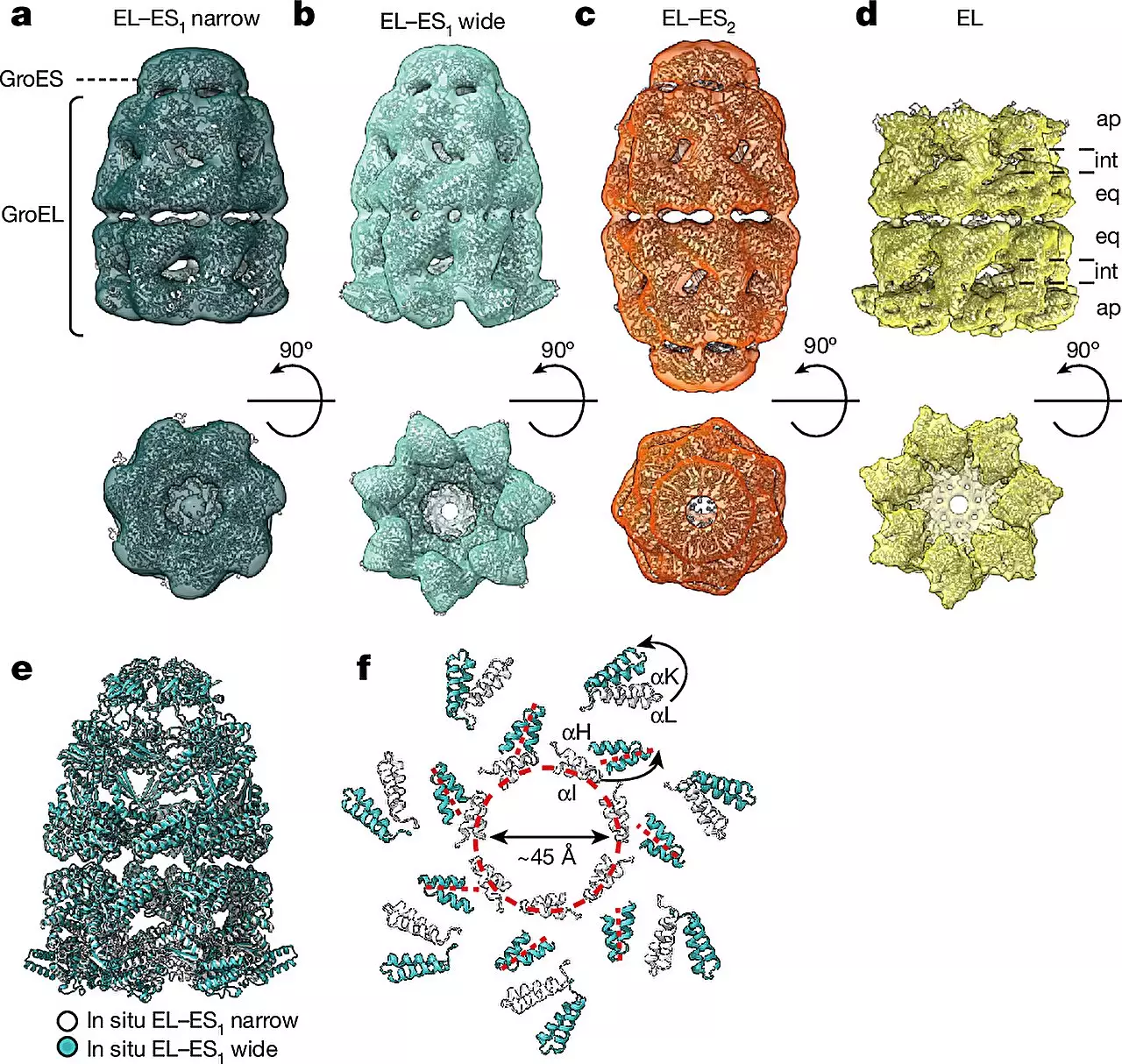
To deepen their comprehension of chaperonins, Hartl collaborated with esteemed structural biologists Wolfgang Baumeister and Rubén Fernández-Busnadiego from the University Medical Center Göttingen. Within bacterial systems, chaperonin complexes consist of GroEL and GroES subunits, with GroEL forming two stacked rings that create a barrel structure while GroES acts as a lid. Notably, the research team observed distinct forms of the GroEL-GroES complex, termed “bullet” and “football,” each displaying unique structural symmetries. The ability to visualize these complexes within live bacterial cells showcases the remarkable advancement facilitated by cryo-electron microscopy in studying complex biological processes.
Fernández-Busnadiego highlights the innovative combination of cryo-ET, single-particle cryo-electron microscopy (cryo-EM), and quantitative mass spectrometry in this study. This interdisciplinary approach enabled the researchers to discern varied conformations of chaperonin complexes under different cellular conditions, shedding light on their abundance and structural dynamics. By conducting these analyses within the native cellular environment, rather than artificial in vitro settings, the research team overcame longstanding challenges associated with studying chaperonin function and mode of action. This breakthrough underscores the pivotal role of cryo-ET in unraveling the intricate mechanisms governing protein folding.
Hartl remarks on the study findings, emphasizing the dynamic nature of chaperonin assembly and the alternating “bullet” and “football” shapes observed during the protein folding process. These insights provide a deeper understanding of how chaperonins facilitate protein folding within live cells, offering a glimpse into the complex orchestration of molecular events critical for cellular function. The integration of advanced technologies and interdisciplinary collaborations continues to drive breakthroughs in the field of structural biology and molecular medicine, paving the way for innovative approaches in disease treatment and drug development.

Leave a Reply