When we think of polymers, the immediate association is with destructive external forces that can weaken the material. However, recent studies have shown that external forces can actually have constructive effects on polymers. By leveraging mechanical forces and flow fields, scientists have been able to impart new functionalities to certain polymers. This field of study, known as mechanochemistry, has opened up new possibilities in polymer synthesis.
A recent study led by Professor Atsushi Shishido from Tokyo Institute of Technology delves into the impact of external forces on the growth behavior of polymers. Published in Macromolecules, the study focuses on how flow fields induced by dynamic UV lighting can influence the process of photopolymerization. This research sheds light on a new and versatile technique for controlling polymer synthesis.
The Key Findings of the Study
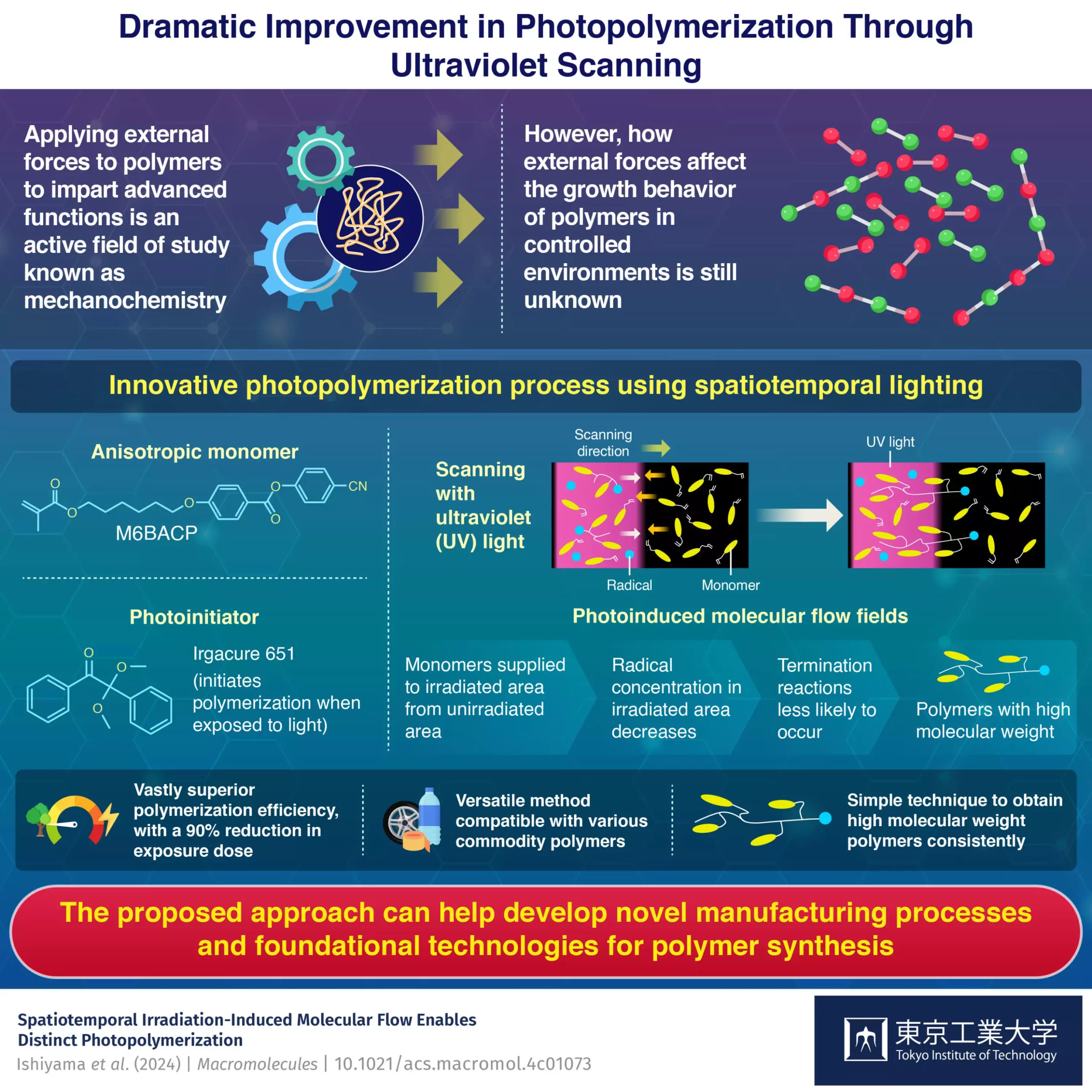
In the study, the researchers utilized M6BACP as a monomer and Irgacure 651 as a photoinitiator in the photopolymerization reaction. Unlike traditional methods, where the entire solution is uniformly irradiated with UV light, the researchers employed a scanning UV light technique. This simple strategy resulted in high molecular weight polymers with a significant reduction in the required exposure dose compared to static uniform light.
The Mechanism Behind the Results
The researchers propose that the movement of UV light induces molecular flows with two main effects. Firstly, it causes growing polymers to diffuse towards the unirradiated area, promoting continuous growth. Secondly, radicals and monomers also diffuse, leading to a lower radical-to-monomer concentration in irradiated areas. This minimizes termination reactions and limits the length of polymer chains.
The findings of this study not only provide valuable insights into photopolymerization reactions but also offer a practical way to enhance industrial processes and polymeric materials. By simply adding movement to the irradiation light, the polymerization efficiency can be significantly improved without altering existing compounds or reaction systems. This breakthrough has the potential to reduce the energy costs associated with photopolymerization and can be applied to various manufacturing processes.
It is worth noting that the advantages observed in the study were not limited to M6BACP but also extended to various commodity polymers like acrylates. These results pave the way for the development of more sustainable methods for manufacturing superior polymers. The impact of mechanochemistry on polymer synthesis is revolutionizing the field and opening up new possibilities for innovation.

Leave a Reply