The intricate structure of bodily carbohydrates, also known as glycans, plays a crucial role in regulating how they attach themselves to other molecules and enzymes. This interaction can have a significant impact on the development and progression of various diseases. Recent research has shed light on how a specific enzyme’s interaction with a small structure in glycans can lead to breakdowns in essential biochemical processes, with potentially severe consequences.
Carbohydrates, known as glycans, are essential components in the attachment process to proteins and lipids in the body. This process, called glycosylation, is involved in a multitude of physiological processes, including cell recognition, cell signaling, immune response, protein folding, development, and fertilization. Any alteration in the structure of glycans can result in the onset or exacerbation of diseases such as cancer, diabetes, Alzheimer’s, and muscular dystrophy.
The field of glycobiology focuses on understanding the role of glycans in various bodily processes and diseases. Despite advancements in identifying the enzymes responsible for glycan production, there is still a need to predict and fine-tune glycan structures within cells more accurately. Enzymes known as glycotransferases play a key role in attaching glycans to proteins or lipids, and their activity must be regulated effectively.
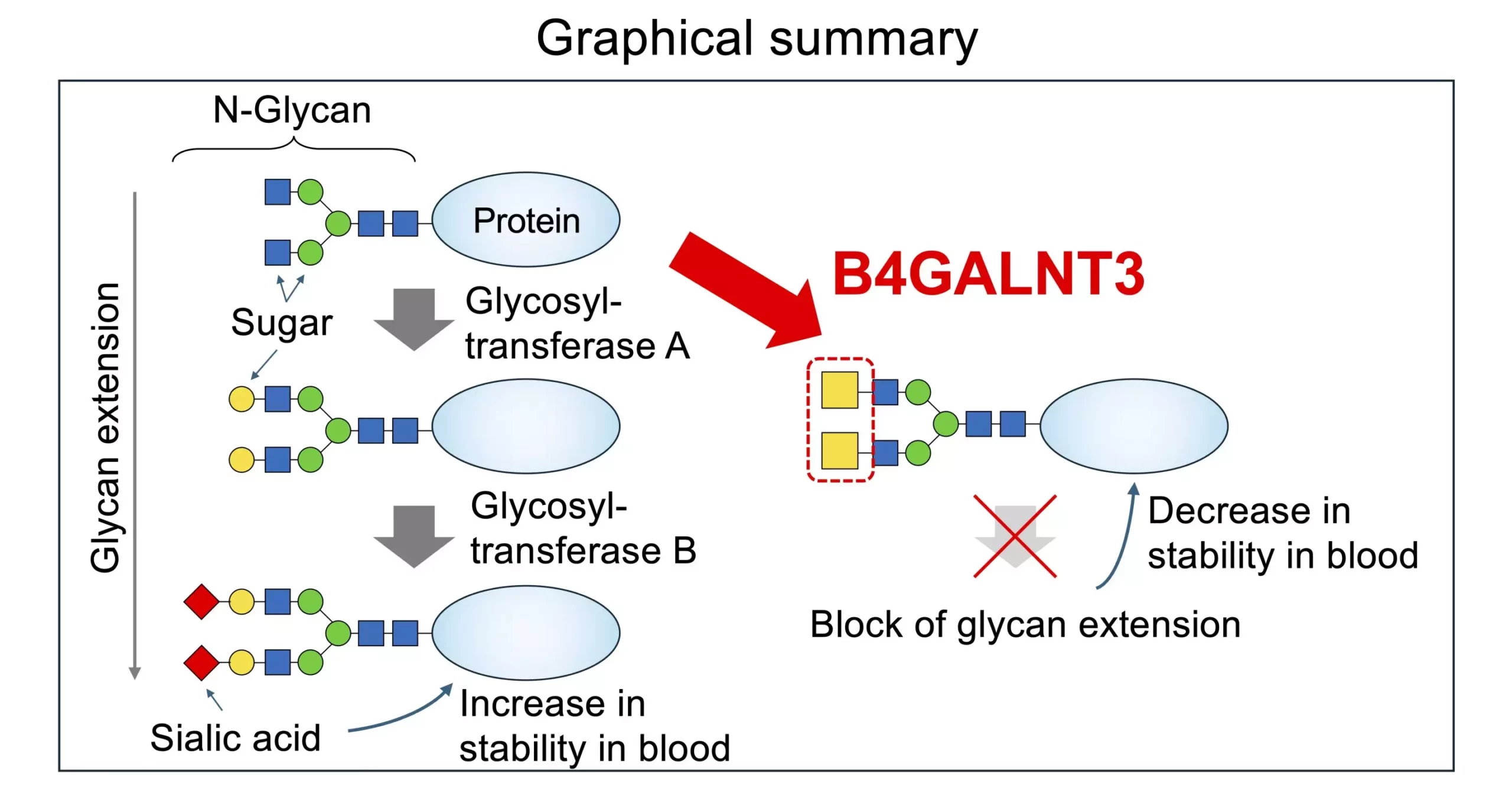
Researchers have discovered a structure in certain types of glycans, such as N-linked and O-linked glycans, known as LacdiNAc or LDN. The biosynthesis of LDN is catalyzed by specific enzymes, B4GALNT3 and B4GALNT4. The presence of LDN is critical for various bodily processes, including hormone modification and stem cell maintenance. Mutations in the enzyme B4GALNT3 have been associated with lower bone density, increased fracture risk, and a higher risk of colon cancer.
Recent advances in structural biology and enzymology have enabled researchers to investigate the interactions between enzymes and glycans on a molecular level. By analyzing the structures of glycosyltransferases, researchers identified a unique feature, the PA14 domain, in B4GALNT enzymes. Mutations in this domain resulted in a significant decrease in enzyme activity, highlighting its essential role in glycan biosynthesis.
The presence of LDN, as opposed to LacNAc, has been shown to influence the actions of glycosyltransferases, particularly in the modification of N-glycans. This terminal modification, known as N-glycan capping, plays a crucial role in determining the structure and function of glycoproteins. Understanding the functional differences between LDN and LacNAc is essential for unraveling the complexities of glycan biosynthesis and its implications for disease pathogenesis.
Researchers aim to further explore the role of B4GALNT3 and LDN in disease development and identify potential therapeutic targets. By delving deeper into the mechanisms underlying enzyme interactions with glycans, scientists can advance our understanding of disease processes and pave the way for targeted interventions to mitigate the impact of malfunctioning glycosylation.
The intricate interplay between glycans, enzymes, and disease pathogenesis underscores the importance of ongoing research in the field of glycobiology. By unraveling the complexities of glycan biosynthesis and enzyme regulation, researchers can gain crucial insights into the mechanisms underlying disease development and progression. The future of glycobiology holds great promise for elucidating the role of glycans in health and disease and developing novel therapeutic strategies to combat a wide range of disorders.

Leave a Reply