Helices are fundamental structural components found in a variety of biological molecules, particularly proteins. These spiral-shaped formations are more than just aesthetic features; they play vital roles in determining how proteins behave and interact within biological environments. The essence of a helix lies in its configuration, specifically in the spatial arrangement of its constituent parts, which significantly influences the properties and functions of the proteins they comprise.
The origin of helical shapes can be traced to peptides, the building blocks of proteins, where the helical formation is a byproduct of the sequential arrangement of amino acids. Each amino acid contributes to a chiral framework—a phenomenon that describes the asymmetric properties of molecules. This chirality is pivotal in the world of biochemistry, as it dictates not only the shape but also the potential interactions of these molecules with other biological systems.
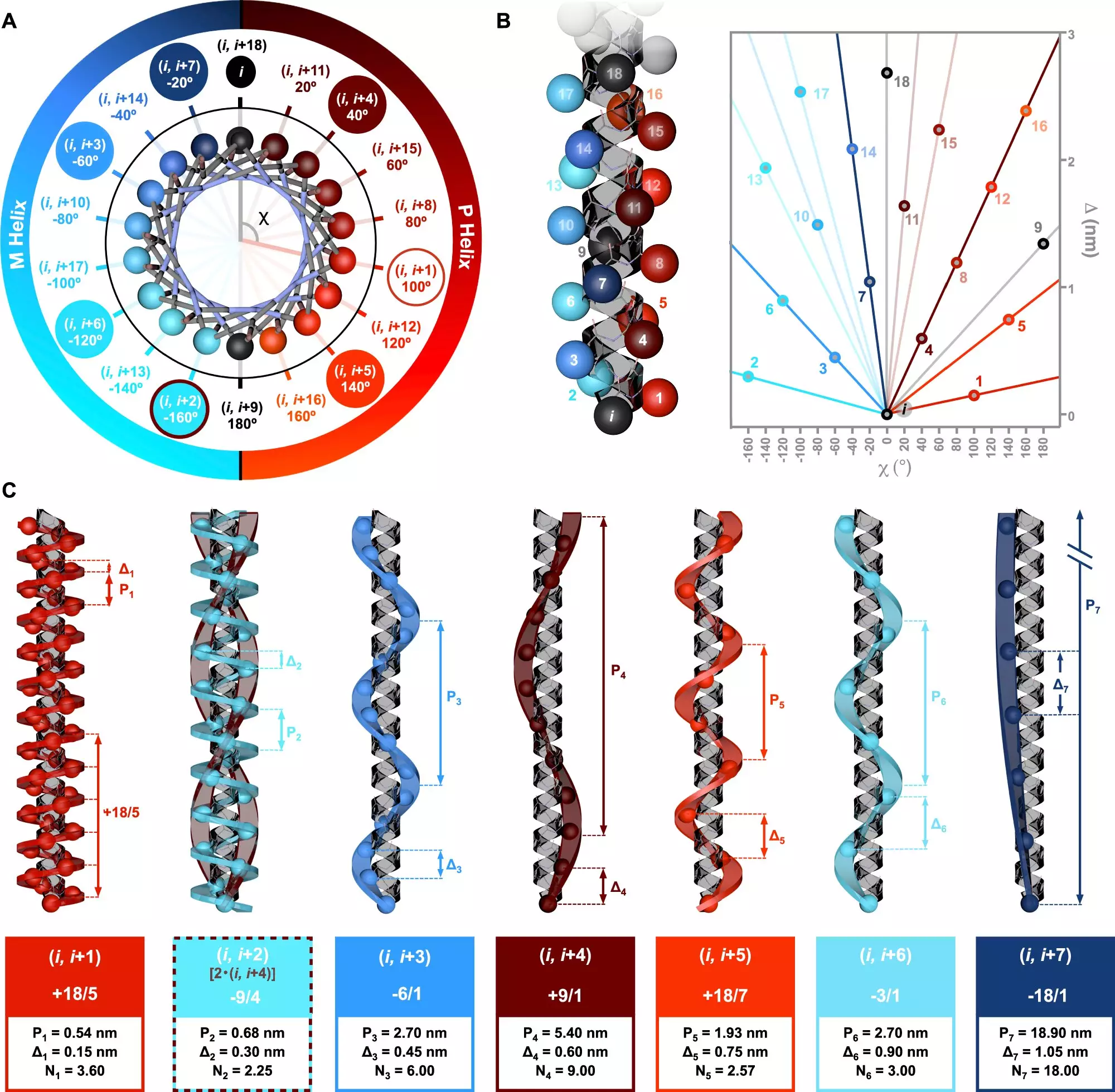
A groundbreaking study recently featured in Nature Communications, spearheaded by Dr. Julián Bergueiro and his team at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS), delves into the intricacies of alpha-helical conformations in peptides. The research illuminates the potential for new layers of chiral complexity that can emerge from these helices. The team identified how various amino acid sequences can lead to distinct helical arrangements in peptides, emphasizing the profound impact sequence variety has on molecular behavior.
Utilizing advanced computational modeling alongside circularly polarized light spectroscopy, the researchers successfully articulated several exo-helical topologies. This dual approach not only allowed them to validate theoretical predictions but also provided a robust framework for understanding the diverse helical formations that can arise from different peptide structures.
The implications of these findings extend far beyond basic science. By elucidating the relationship between amino acid sequences and helical structures, this research lays the groundwork for innovative applications in medicinal chemistry and biotechnology. As Dr. Bergueiro affirms, manipulating the order of amino acids could lead to the design of specific helical conformations along polymer chains, thereby allowing for tailored functionalities in new biocompatible materials and pharmaceuticals.
This approach represents a transformative capability in macromolecular engineering, providing tools to construct bespoke molecules that cater to specific therapeutic needs or functional requirements. The capacity to design new compounds with precise helical characteristics positions researchers at the forefront of advancements in drug design and material science.
The exploration of helical structures within peptides is not merely a technical study; it is a pivotal step forward in our understanding of protein functionality and synthesis. As researchers continue to uncover the complexities of these structures and their chiral characteristics, we stand on the brink of numerous scientific advancements. The ability to engineer macromolecules with targeted properties heralds a new era in peptide chemistry, promising breakthroughs that could revolutionize healthcare, biotechnology, and beyond.

Leave a Reply