In an era where environmental degradation is a pressing concern, innovative approaches to remove pollutants, especially micropollutants like pesticides and trace chemicals, have become essential. These tiny contaminants pose significant risks to aquatic ecosystems and, ultimately, human health. One groundbreaking technique emerging in this domain is photocatalysis, a method that employs semiconducting nanomaterials and sunlight. A recent study from Cornell University illustrates the potential of this approach, revealing intriguing insights into how the combination of titanium dioxide (TiO2) and gold co-catalysts can enhance the removal of these harmful substances.
Understanding Photocatalysis: The Mechanics Behind the Method
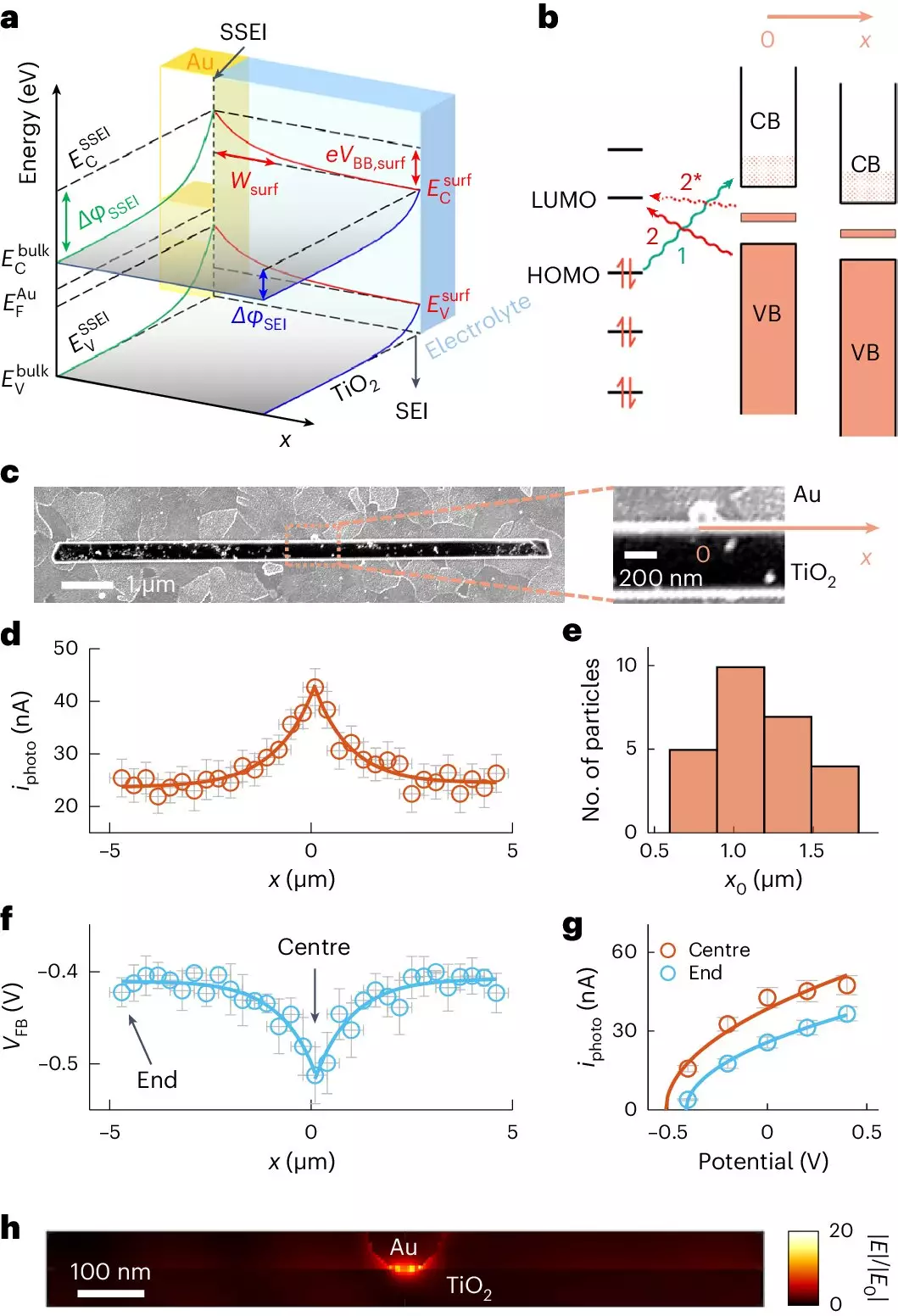
Photocatalysis works by utilizing sunlight to activate a catalyst, in this case, titanium dioxide, which interacts with pollutants on a molecular level. The idea is to create a reactive environment where toxic substances adhere to the catalyst’s surface and are subsequently broken down. The research team at Cornell has unveiled a superior method for examining how pollutants attach to TiO2 surfaces, revealing that adding gold particles significantly elevates the efficiency of this process.
The newly developed technique, known as adCOMPEITS (Adsorption-based COMPetition Enabled Imaging Technique with Super-resolution), allows scientists to visualize adsorption dynamics with unprecedented clarity. By employing a fluorescent probe molecule, the researchers could monitor how well pollutants like the pesticide pirimiphos methyl and diethyl phthalate adhered to the TiO2 surface in real time, thereby creating a detailed map of adsorption behavior that elucidates the underlying mechanisms.
The Role of Gold: More Than Just a Catalyst
What’s particularly remarkable about this study is the role of gold. Traditionally regarded as a luxury material, gold nanoparticles, measuring just 100 nanometers, have shown the ability to not only boost the adsorption capacity of TiO2 but also extend its influence over micrometer distances. It was found that the gold particles induce a change in the electronic properties of the TiO2 surface, a phenomenon known as surface band bending.
This band bending alters the electronic landscape of the semiconductor, extending the zone of influence far beyond the immediate vicinity of the gold nanoparticles. The researchers discovered that the enhancement in adsorption could occur as much as ten times farther away than previously understood. This groundbreaking revelation suggests that only minimal amounts of gold are necessary to achieve pronounced effects, making the process not only innovative but also economical.
A Broader Implication: Advancing Photocatalytic Efficiency
The implications of this research extend beyond simply improving the removal of micropollutants. The challenge of low conversion efficiency in photocatalysis—where solar energy is converted into a chemical reaction—could see considerable advancements through the strategic use of metal nanoparticles like gold. As the Cornell team asserts, this long-range enhancement could catalyze developments in myriad applications, from environmental cleanup to advancements in solar energy technologies and chemical sensing.
Moreover, understanding the dynamics of adsorption in such systems is pivotal for designing more efficient photocatalytic materials. The integration of nanotechnology, especially the use of metal nanoparticles, symbolizes a decisive shift in how we can approach treating contaminated water sources. By enhancing the efficiency of photocatalysis, we pave the way for scalable and sustainable methods for combating environmental pollutants.
The Future of Environmental Chemistry: Towards Sustainability
As the field of photocatalysis continues to evolve, the potential for integrating these findings into practical applications grows exponentially. The implications of this research are not just limited to academic curiosity; they breathe new life into the ongoing quest for sustainable environmental solutions. By deploying refined photocatalytic systems powered by sunlight, we can significantly mitigate the adverse effects of micropollutants on our environment.
The work conducted by the Cornell team epitomizes the convergence of nanotechnology and environmental chemistry. The ongoing exploration of novel materials and methods will surely bolster our capacity to safeguard public health and the integrity of our ecosystems. This exciting frontier holds the promise of a cleaner, greener future, fueled by the insights gleaned from innovative research in photocatalytic technology.

Leave a Reply