The recent discovery by a team of researchers led by Professor Magnus Wolf-Watz from Umeå University has illuminated the crucial role of magnesium in catalyzing the synthesis of adenosine triphosphate (ATP), the primary energy currency in biological cells. This groundbreaking study, featured in the journal Science Advances, uncovers the intricate details of how magnesium atoms orchestrate the spatial configuration of molecules involved in ATP production. ATP serves as a universal energy carrier, facilitating essential processes from muscular contractions to the transport of molecules across cell membranes. Understanding the mechanics of ATP synthesis not only reveals insights into fundamental biology but also has implications for medical research, particularly in fields concerning metabolic diseases and infections.
ATP synthesis is governed by a pivotal enzyme known as adenylate kinase. This enzyme catalyzes the reversible conversion of adenosine diphosphate (ADP) and adenosine monophosphate (AMP) into ATP. Previous research has established that magnesium is integral to this process, primarily by providing the necessary electrostatic environment that accelerates chemical reactions involving ATP. However, the speed required for ATP generation is not solely a function of electrostatics; the precise alignment of the substrates ADP and AMP within the enzyme’s active site is equally critical. This vital spatial arrangement allows for efficient catalysis, a facet that this recent study has explored in depth.
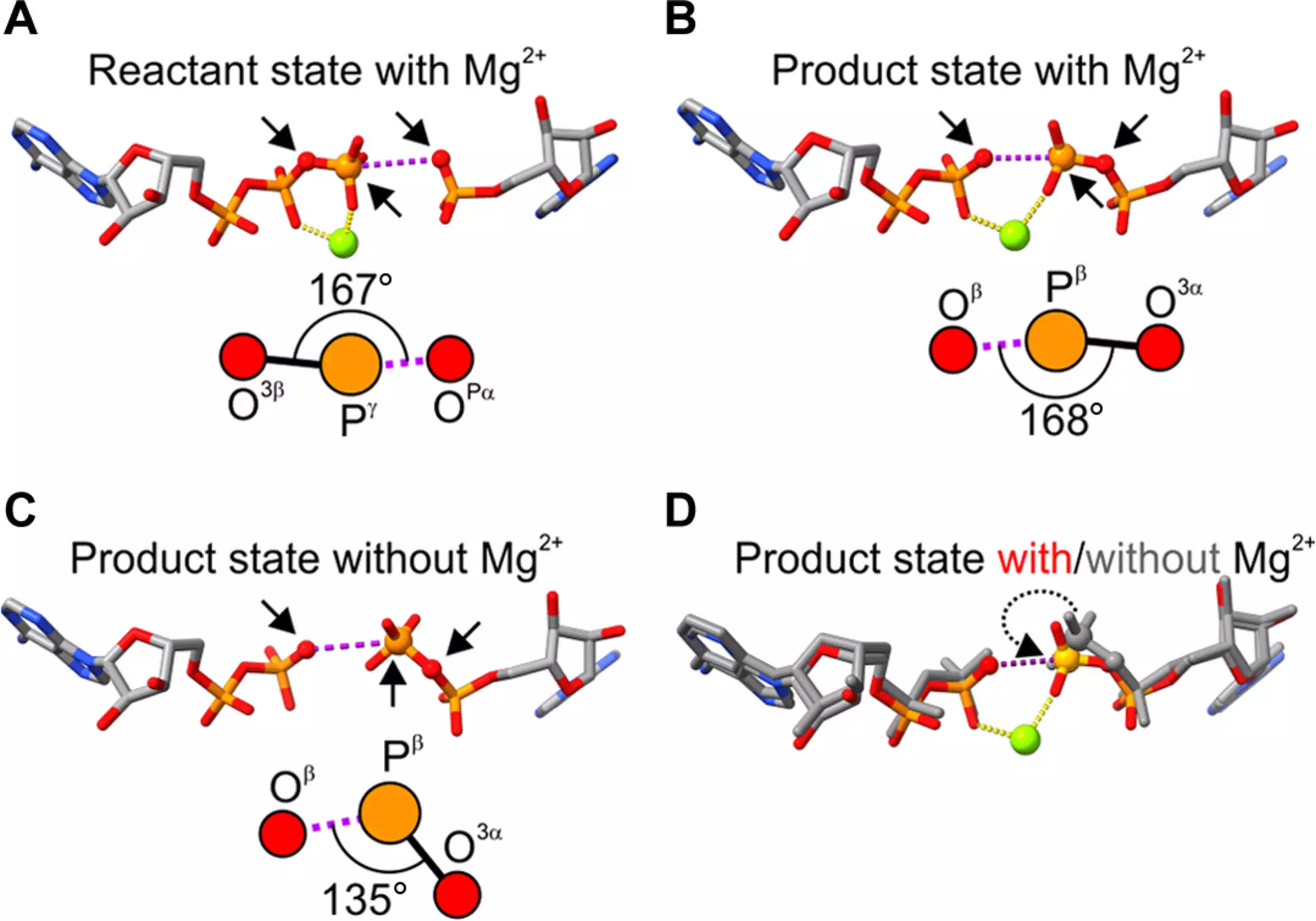
The research team embarked on a detailed investigation into how magnesium ions influence the geometry of ADP and AMP in the active site of adenylate kinase. Their findings indicated that the magnesium atom not only facilitates the chemical reactions but also plays a geometrical role by adjusting the angle of the substrates. This angle adjustment ensures that the substrates attain the optimal configuration necessary for efficient catalysis. Professor Wolf-Watz referred to this discovery as “astonishing,” highlighting how minor molecular alterations can lead to significant enhancements in enzymatic activity. The revelation that magnesium can direct structural changes within the enzyme opens a new frontier in understanding catalytic mechanisms.
The research approach employed by the team was multifaceted. The team utilized crystallographic structures produced by Professor Elisabeth Sauer-Eriksson, providing a visual representation of the enzyme and its interactions with magnesium. This experimental data was complemented by computational chemistry techniques from Kwangho Nam’s lab at the University of Texas at Arlington. By merging experimental and computational methods, the researchers identified a correlation between the angular changes induced by magnesium and broader structural adjustments within the enzyme. This integration of practices showcases the benefits of interdisciplinary collaboration in advancing our understanding of biochemical processes.
The implications of these findings extend far beyond basic science. With ATP being central to numerous metabolic pathways, elucidating the mechanics of its synthesis could inform therapeutic strategies for a variety of health conditions. For instance, a deeper understanding of ATP production could provide insights into the treatment of diseases characterized by energy metabolism dysfunction, such as diabetes or ischemia. Furthermore, the study could pave the way for advanced research into bacterial infections, where ATP plays a critical role in pathogen viability and virulence.
The research led by Professor Wolf-Watz and his team represents a significant step forward in our comprehension of enzyme catalysis and the role of metal ions in biochemical reactions. By demonstrating how magnesium not only aids in the creation of ATP but also modulates the geometric arrangement of reactants, this study contributes to a holistic view of enzymatic function. As we continue to explore the intricate web of life at the molecular level, discoveries like these serve as reminders of the fascinating complexity that sustains biological systems. This newfound knowledge not only enriches the scientific community’s resources but also invites further exploration into the profound chemistry that underlies life itself.

Leave a Reply