The relentless pursuit of more efficient catalysts has led researchers to explore the nuances of single-atom catalysis. A notable contribution to this field comes from a groundbreaking study spearheaded by Prof. Yan Wensheng’s team at the University of Science and Technology of China (USTC). They have delved into the complexities of metal loading and its impact on catalytic activity, specifically in the area of acidic oxygen evolution reactions (OER). The implications of this research are timely and critical as industry and science alike push for greener and more sustainable catalytic processes.
Decoding the Challenge
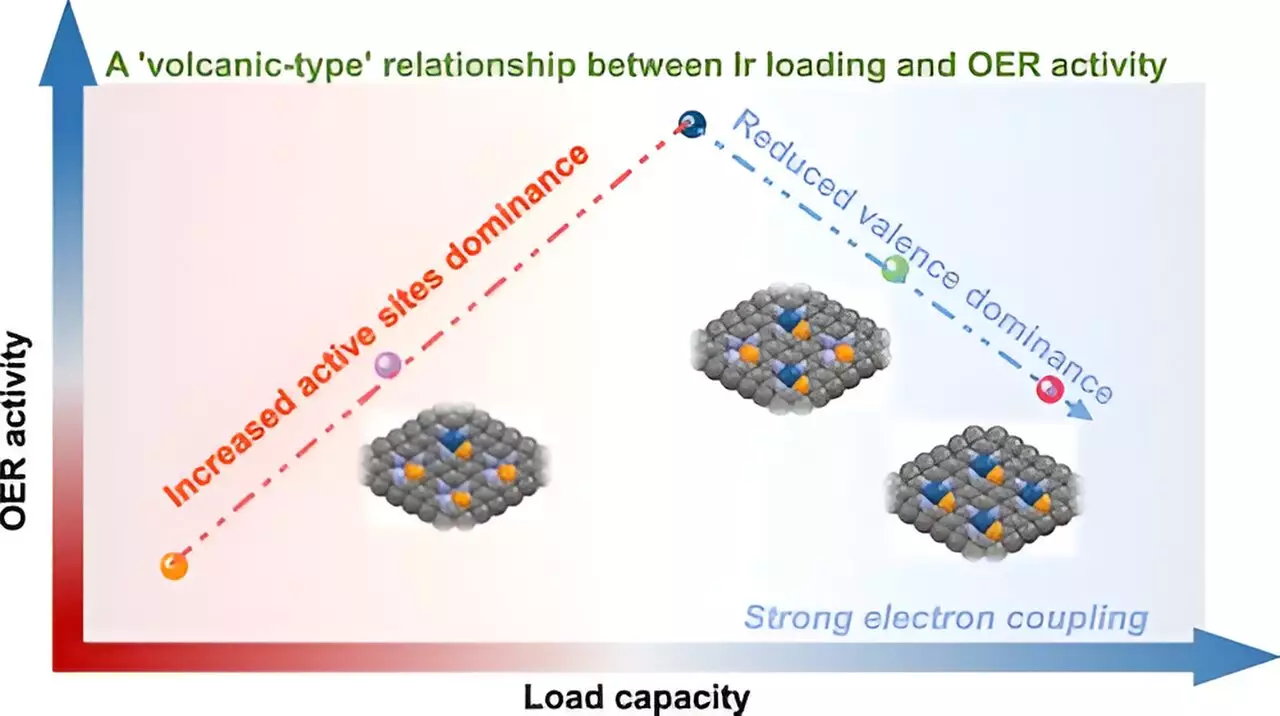
One major challenge in the realm of single-atom catalysts is designing materials that can maintain high metal loading while avoiding issues like aggregation—where individual metal atoms cluster together, diminishing overall effectiveness. The research highlights a significant breakthrough with the development of “volcano-type” relationship insights, advocating for a more sophisticated understanding of metal interactions at atomic levels. Such insights are vital for crafting catalysts that not only maximize efficiency but also provide flexibility in application across various chemical processes.
A Quantum Leap in Metal Coordination
The researchers utilized a novel P-anchoring strategy which enabled them to synthesize Ir single-atom catalysts with impressive metal loadings ranging from 5% to 21 wt%. This is particularly intriguing because conventional wisdom often assumed that increasing metal loading would linearly enhance catalytic activity. However, the study unveiled a more complex reality. The strength and stability of the Ir-P coordination structure was identified as crucial in preventing the aggregation of Ir atoms, thereby promoting an effective interaction between the atomic and molecular structures involved in the OER processes.
The Volcano-Type Relationship Explained
Through advanced methodologies including synchrotron radiation X-ray Absorption Spectroscopy (XAS) and X-ray Photoelectron Spectroscopy (XPS), the researchers painted a clearer picture of how metal loading influences catalytic behavior. The so-called “volcano-type” relationship was defined by a peak: increased Ir loading initially enhances the number of available active sites for the catalytic reaction. Yet, once the load surpasses a specific threshold, counterproductive interactions between adjacent Ir atoms crystallize into diminished catalytic performance. This dual-complexity illustrates that more is not always better—a lesson that could reshape conventional wisdom surrounding catalyst design.
Implications for Future Research
This pioneering work provides not just theoretical strategies for developing more efficient single-atom catalysts, but also a broader understanding of how atomic interactions dictate reaction outcomes. Researchers now have a clearer avenue for innovation, paving the way for catalysts that are not only potent but also economically viable. The findings urge a rethink of catalyst optimization and underscore the importance of balancing between high metal loading and functional efficiency. As the urgency for sustainable solutions grows, encapsulating these insights in future designs may very well revolutionize the field of catalysis, enhancing our ability to tackle environmental challenges head-on.

Leave a Reply