Aluminum oxide, known scientifically as Al2O3, has intrigued scientists and engineers for decades. This versatile compound, commonly recognized as alumina, corundum, sapphire, or even ruby, serves critical roles across various industries. Its remarkable insulating properties enable applications in electronics, catalysis, and ceramics, making it a pivotal material in modern technology. However, understanding the surface characteristics of aluminum oxide has remained elusive for over fifty years, primarily due to its insulating nature. Recent advancements have finally illuminated the structure of this enigmatic surface, addressing a long-standing mystery in the realm of surface science.
Surface structures play a pivotal role in determining the chemical reactivity of materials, particularly in catalytic processes. The precise arrangement of atoms on a material’s surface can significantly influence how it interacts with reactants, thereby affecting the efficiency of catalytic activities. For aluminum oxide, the challenge has been that while the internal atomic structure adheres to a predictable arrangement—leading to its characteristic crystalline shapes—the atoms on the surface display a more complex and less understood configuration. The past decades saw this surface structure classified as one of the “three mysteries of surface science,” complicating our understanding of its properties and applications.
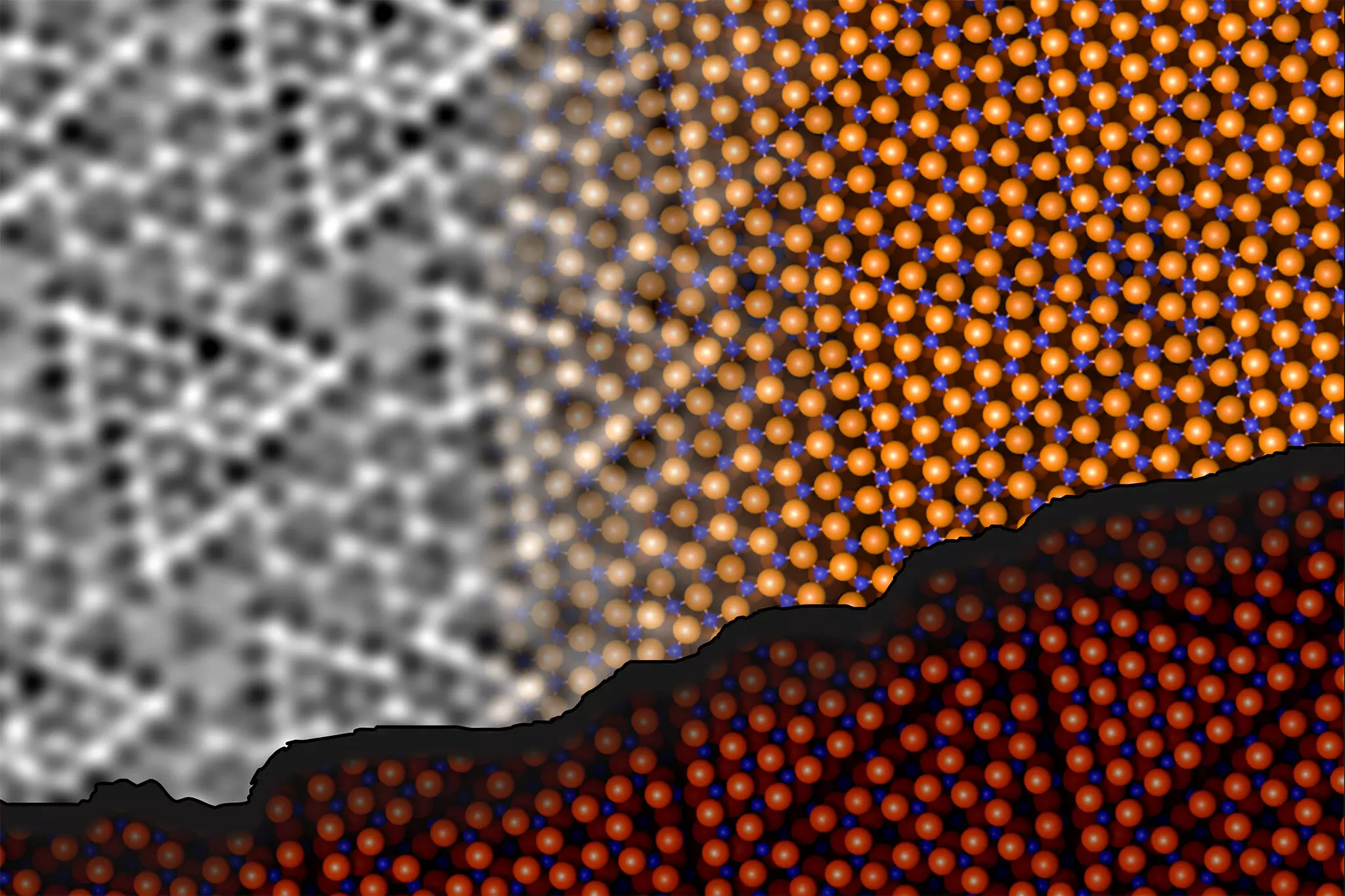
A dedicated research team from TU Wien and the University of Vienna has successfully cracked the complex structure of the aluminum oxide surface. Led by Jan Balajka and Ulrike Diebold, this team has employed advanced techniques, namely non-contact atomic force microscopy (ncAFM), to probe the surface conformation of Al2O3. This innovative method involves scanning a finely tuned tip above the material’s surface, allowing for the high-resolution visualization of atomic arrangements without causing physical contact.
The implications of this technique are profound. According to researcher Johanna Hütner, the ncAFM images depict atomic locations, albeit without revealing their chemical identities. To navigate this limitation, the researchers ingeniously modified the ncAFM tool by attaching a single oxygen atom to the tip. This alteration enabled them to discern the chemical identity of surface atoms, mapping the areas of attraction and repulsion between the tip and the surface atoms.
The groundbreaking findings indicate that the aluminum atoms at the surface engage in interactions with oxygen atoms located within deeper layers of the material. This penetration enables the formation of chemical bonds and results in a structural rearrangement of the first two atomic layers. Surprisingly, while this restructuring minimizes energy and stabilizes the surface, the overall ratio of aluminum to oxygen remains consistent with previous understandings. This insight contradicts earlier assumptions regarding surface behavior uniformly, shedding light on the complexities inherent to aluminum oxide.
In concert with experimental advancements, computational modeling played a crucial role in elucidating the surface structure. The researchers optimized a three-dimensional model of the aluminum oxide surface utilizing state-of-the-art machine learning techniques. Andrea Conti, who spearheaded the computational aspects, noted the vast possibilities for the arrangement of atoms beneath the surface, which complicated the modeling process. The combination of sophisticated algorithms and traditional computational methods allowed the team to tackle this complexity effectively, ultimately culminating in the creation of a stable model representative of the surface architecture.
The successful determination of aluminum oxide’s surface structure not only resolves a long-standing mystery but also opens doors to advancements across multiple domains. Jan Balajka emphasizes that the findings establish fundamental design principles applicable to various materials, promising improvements in catalytic efficiency, material science, and beyond. By demystifying this vital insulator, researchers can now explore novel applications and optimize existing technologies, enhancing the effectiveness of catalysts and the performance of electronic devices.
The exploration of aluminum oxide’s surface has illuminated critical insights into the molecular world that underlies many modern systems. This research paves the way for future innovations, showcasing the importance of interdisciplinary collaboration in solving complex scientific puzzles and advancing technology.

Leave a Reply