Noble gases, known for their lack of reactivity and stability, have long been considered inert elements in the periodic table. Traditionally, this perception has limited our understanding of their potential interactions. However, over sixty years ago, a groundbreaking experiment by Neil Bartlett challenged this notion by successfully synthesizing a compound containing xenon, opening new doors in the field of chemistry. Despite this initial success, studying noble gas compounds remains a complex endeavor due to the challenges associated with their stable crystal growth and structures.
Bartlett’s creation of xenon hexafluoroplatinate (XePtF6) was not just a milestone; it was commemorated with an International Historic Chemical Landmark. The discovery sparked significant interest, leading to the synthesis of hundreds of noble gas compounds. Yet, researchers face formidable obstacles when attempting to analyze these compounds further. The inherent sensitivity to moisture in the air complicates the growth of large crystals, posing a barrier to detailed structural analysis using traditional methods such as single-crystal X-ray diffraction.
In a recent study published in ACS Central Science, researchers have tackled these challenges head-on. They employed a novel technique—3D electron diffraction—to analyze small crystallites of xenon compounds. This fresh approach not only allows for the examination of tiny samples that exhibit stability in air but also pushes the boundaries of what can be achieved with delicate air-sensitive materials. Previous methodologies had not focused on this area, leaving many xenon compounds unexplained.
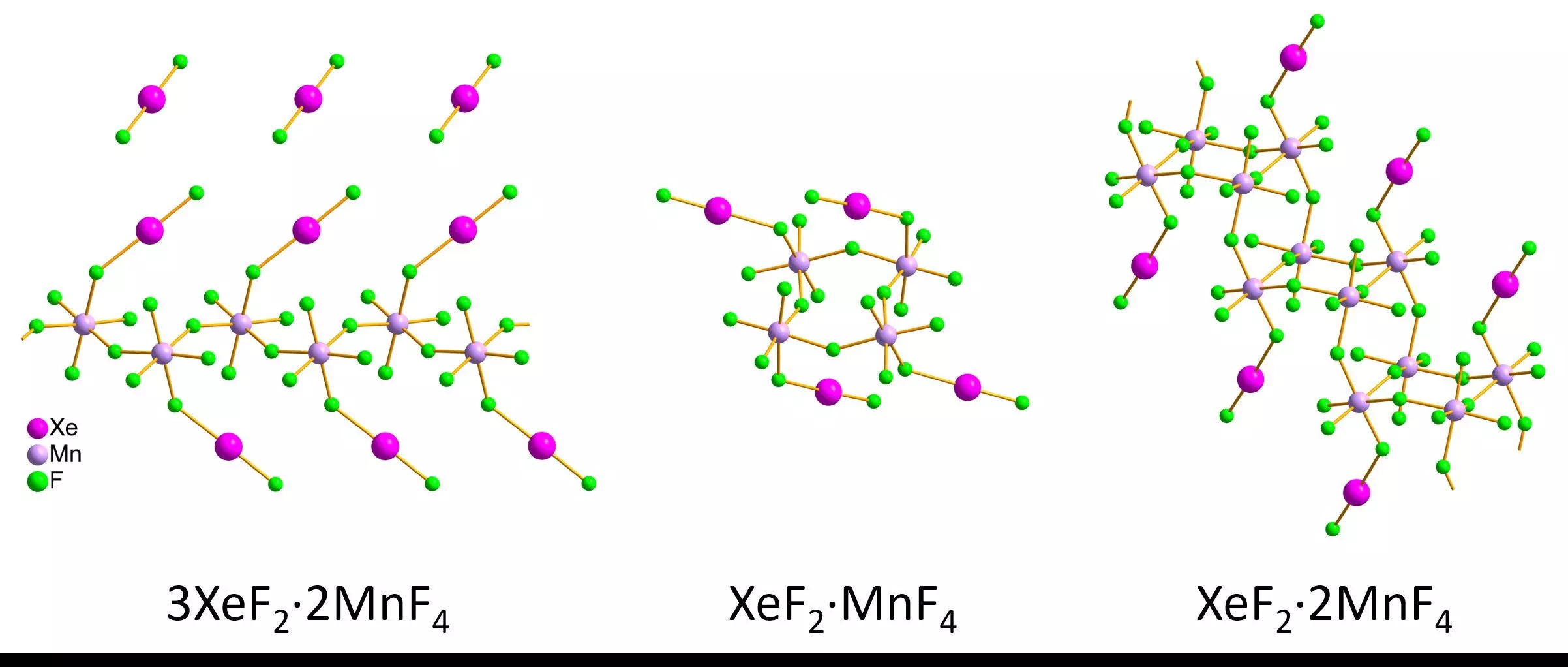
The researchers, led by Lukáš Palatinus and Matic Lozinšek, meticulously constructed xenon difluoride-manganese tetrafluoride compounds, yielding both individual red crystals and pink crystals in powder form. By implementing advanced cooling techniques and protective measures during the transfer to a transmission electron microscope, the team ensured sample integrity for accurate measurement.
Through their diligent work, the team was able to measure critical bond lengths and angles in xenon-fluoride and manganese-fluoride compounds. Their findings revealed structural variations among the compounds that had not been previously characterized. Observations included infinite zigzag chains, ring formations, and intricate staircase-like double chains. The correlation between the results from 3D electron diffraction and traditional X-ray diffraction methods was notable, providing a new layer of confidence in this innovative technique.
Furthermore, this research demonstrates the potential of 3D electron diffraction to characterize a wider array of noble gas compounds that have remained enigmatic for decades, such as XePtF6. The success of this study not only paves the way for a deeper understanding of noble gases but also establishes a precedent for studying other air-sensitive materials.
The evolution of research methods and techniques in the study of noble gases is not just a testament to human ingenuity but also a reminder of the complexity and richness of chemical interactions. As we continue to explore the structures and properties of noble gas compounds, emerging methodologies like 3D electron diffraction will undoubtedly lead to further breakthroughs, challenging our understanding and expanding the boundaries of chemical science.

Leave a Reply