Ice has long been suspected to have played a significant role in the emergence of life on Earth. The presence of orderly arranged water molecules in ice creates gaps in the crystal lattice where organic molecules can be concentrated. This concentration of organic compounds within ice has sparked interest in studying the interactions between water ice and organic molecules. However, traditional methods of studying organic molecules in ice, such as Raman and infrared spectroscopy, have their limitations, particularly in terms of measurement sensitivity.
Recently, a research team led by Prof. Zhang Guoqing, Prof. Liu Shiyong, Prof. Zhou Xiaoguo, and Researcher Zhang Xuepeng from the University of Science and Technology of China (USTC) developed a novel method for detecting water-ice microstructures using organic phosphorescent probes and phosphorescence spectroscopy. Their research, published in Angewandte Chemie International Edition, focuses on utilizing emission-based techniques to study organic molecules within water ice.
The team’s study demonstrates how the microstructures of water ice can be influenced by trace amounts of water-soluble organic molecules. By employing a phosphorescent probe called acridinium iodide (ADI), the researchers were able to differentiate between amorphous and crystalline water ice. In amorphous ice, the ADI probe exhibits long-lived phosphorescence, producing a visible greenish-yellow afterglow. In contrast, ordered crystalline ice induces short-lived red phosphorescence due to the aggregation of ADI probe molecules.
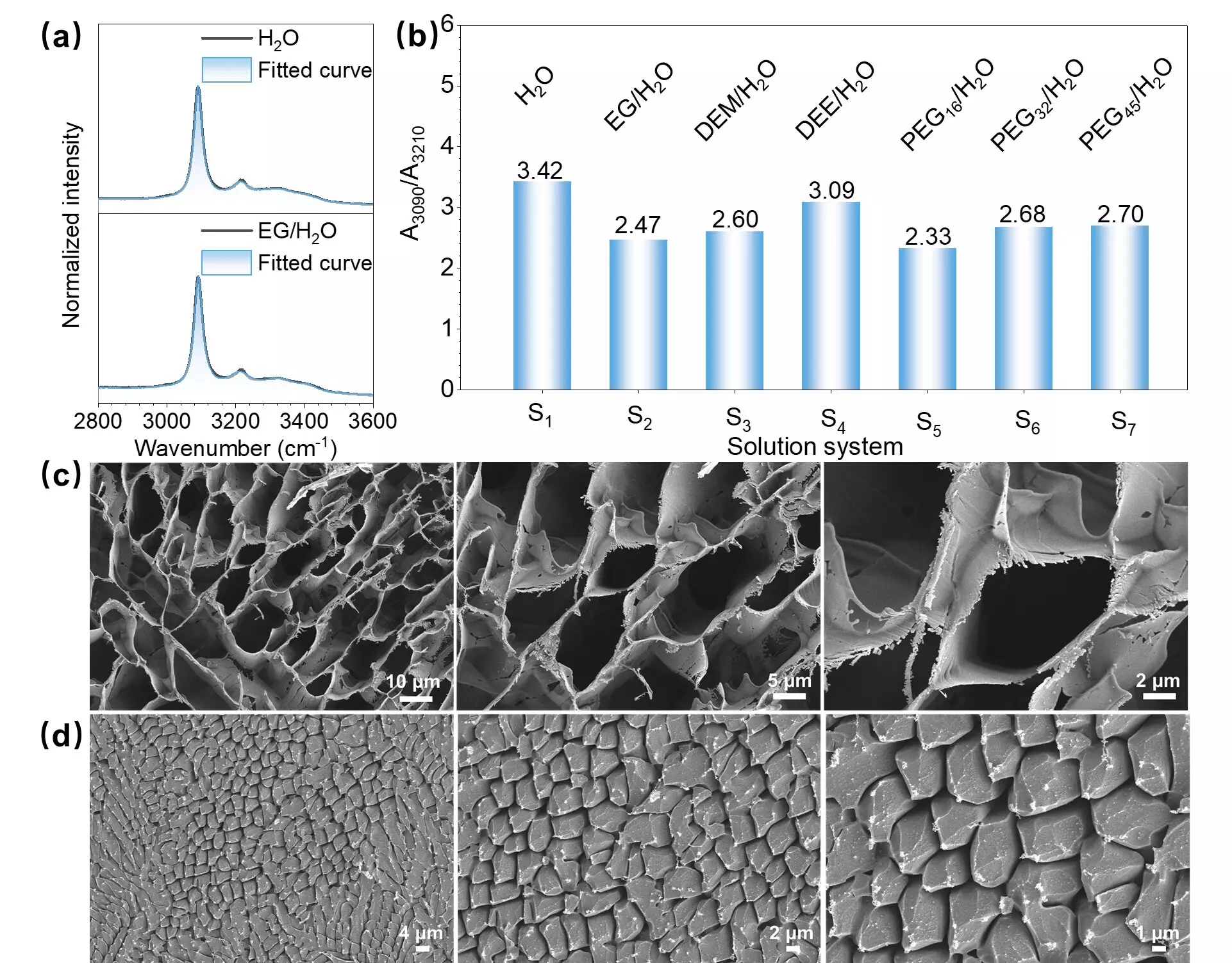
The team’s experiments revealed significant spectroscopic changes when ethylene glycol (EG) small molecules and monodispersed EG polymers were added to water ice containing ADI. The addition of trace amounts of EG led to the transformation of ADI molecules in water ice from undissolved aggregates to dissolved ion states. This transformation was evident through changes in the fluorescence and phosphorescence bands, with distinct vibronic progressions observed in the emission spectra.
Corroborating Results
To validate their findings, the researchers utilized low-temperature scanning electron microscopy (Cryo-SEM) and low-temperature Raman (LT-Raman) spectroscopy. Cryo-SEM images showed that the addition of trace EG into water ice containing ADI resulted in local areas with porous microstructures. The LT-Raman spectra confirmed a shift in the O-H vibration of water ice from a crystalline state to a glassy state upon the addition of trace EG.
Overall, this study highlights the inhibitory effect of trace organic molecules on the crystalline order of water ice and provides a more sensitive means of studying water-ice-organics interactions through phosphorescence spectroscopy. The ability of phosphorescence spectroscopy to reveal morphological differences in water-ice microstructures when different organic molecules are added further enhances our understanding of these complex interactions.
The research conducted by Prof. Zhang Guoqing and his team sheds light on the intricate relationship between water ice, organic molecules, and the emergence of life. By developing a new method for studying organic molecules in ice, the research opens up exciting possibilities for further exploration in this field.

Leave a Reply